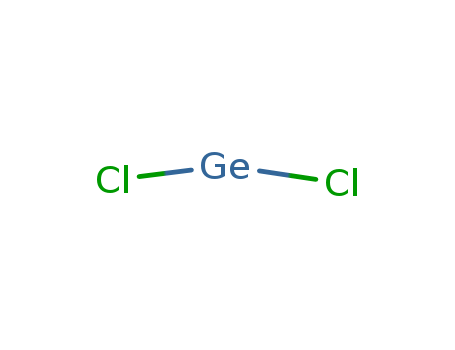
- Chemical Name:Unii-SI7jkx4F22
- CAS No.:10060-11-4
- Molecular Formula:Cl2Ge
- Molecular Weight:143.496
- Hs Code.:
- European Community (EC) Number:233-192-1
- DSSTox Substance ID:DTXSID40143425,DTXSID80164992
- Wikidata:Q2331626
- Mol file:10060-11-4.mol
Synonyms:10060-11-4;GeCl2;UNII-SI7JKX4F22;EINECS 233-192-1;Cl2Ge;Cl2-Ge;DTXSID40143425;DTXSID80164992;CID 6327122;Germanium chloride (GeCl2) (6CI,8CI,9CI);Q2331626