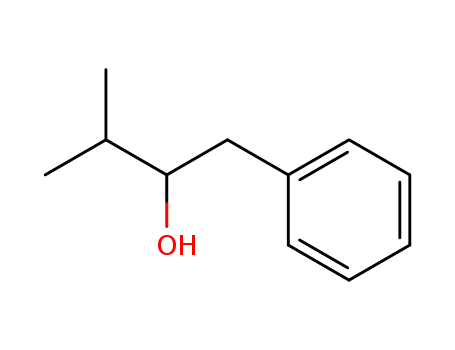
- Chemical Name:3-Methyl-1-phenylbutan-2-ol
- CAS No.:705-58-8
- Deprecated CAS:120442-22-0
- Molecular Formula:C11H16 O
- Molecular Weight:164.247
- Hs Code.:
- European Community (EC) Number:211-886-5
- NSC Number:68518
- UNII:N06AQ6J88Y
- DSSTox Substance ID:DTXSID00862382
- Nikkaji Number:J28.643E
- Wikidata:Q27284333
- Mol file:705-58-8.mol
Synonyms:3-methyl-1-phenylbutan-2-ol