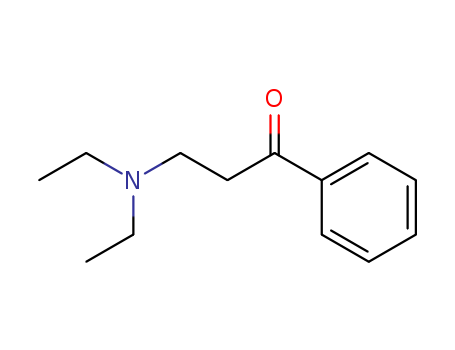
- Chemical Name:3-(Diethylamino)propiophenone
- CAS No.:94-38-2
- Molecular Formula:C13H19 N O
- Molecular Weight:205.3
- Hs Code.:2922399090
- European Community (EC) Number:202-329-7
- NSC Number:287355
- UNII:8SBE3AMK5D
- DSSTox Substance ID:DTXSID60240187
- Nikkaji Number:J182.102D
- Wikidata:Q83122916
- ChEMBL ID:CHEMBL130526
- Mol file:94-38-2.mol
Synonyms:3-(diethylamino)-1-phenylpropan-1-one;94-38-2;3-(Diethylamino)propiophenone;1-Propanone, 3-(diethylamino)-1-phenyl-;Diethylaminopropiophenone;Propiophenone, 3-(diethylamino)-;3-Diethylaminopropiophenone;3-Diethylamino-1-phenyl-propan-1-one;NSC 287355;CHEMBL130526;EINECS 202-329-7;NSC287355;.beta.-N,N'-Diethylaminopropiophenone;NSC 322068;NSC-287355;3-(Diethylamino)-1-phenyl-1-propanone;NSC322068;8SBE3AMK5D;3-(diethylamino)propiophenon;SCHEMBL6322827;DTXSID60240187;BDBM50036715;AKOS005258684;SB78555;1-Phenyl-3-(diethylamino)-1-propanone;2-(N,N-Diethylamino)ethyl phenyl ketone;3-(diethylamino)-1-phenyl-propan-1-one;1-Propanone,3-(diethylamino)-1-phenyl-;NCI60_002793;CS-0001980;FT-0646744;EN300-186867;J-510863;Z993668806;Ethanone, 1-(2,4-dibromophenyl)-2-(1H-1,2,4-triazol-1-yl)-