- Chemical Name:Orthoperiodic acid
- CAS No.:10450-60-9
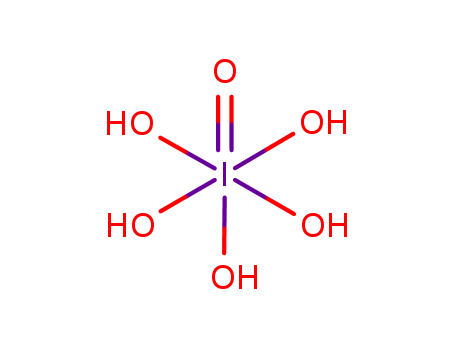
- Molecular Formula:H5IO6
- Molecular Weight:227.941
- Hs Code.:
- European Community (EC) Number:233-937-0
- UNII:AK1D44L87G
- DSSTox Substance ID:DTXSID10883144
- Nikkaji Number:J96.462J
- Wikidata:Q411297
- Mol file:10450-60-9.mol
Synonyms:Acid, Paraperiodic;Acid, Periodic;Acids, Periodic;Paraperiodic Acid;Periodic Acid;Periodic Acid (HIO4);Periodic Acids


 C,
C, O
O
 O:Oxidizing agent;
O:Oxidizing agent;

