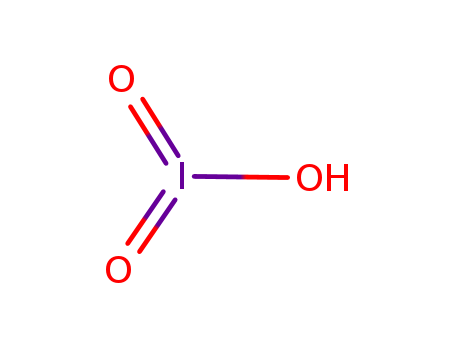
- Chemical Name:Iodic acid
- CAS No.:7782-68-5
- Deprecated CAS:13453-60-6
- Molecular Formula:HIO3
- Molecular Weight:175.911
- Hs Code.:28111980
- European Community (EC) Number:231-962-1
- UNII:6U8J18JSBM
- DSSTox Substance ID:DTXSID2064812
- Nikkaji Number:J43.589I
- Wikipedia:Iodic acid
- Wikidata:Q408822,Q27110316
- ChEMBL ID:CHEMBL1161636
- Mol file:7782-68-5.mol
Synonyms:iodic acid;iodic acid, 131I-labeled;iodic acid, lead (2+) salt;iodic acid, lithium salt;iodic acid, magnesium salt;iodic acid, mercury (2+) salt;iodic acid, potassium (2:1) salt;iodic acid, rubidium salt;iodic acid, sodium salt, 125I-labeled;iodic acid, strontium salt


 O,
O, C
C


