10.1002/ejoc.200600968
The research investigates the impact of ring substituents and leaving groups on the kinetics of SNAr (Nucleophilic Aromatic Substitution) reactions involving 1-halogeno- and 1-phenoxy-nitrobenzenes with aliphatic amines in acetonitrile. The study reports rate constants for the reactions, comparing them with previously reported data for more strongly activated compounds. The reactants included a series of 1-chloro-, 1-fluoro-, and 1-phenoxy-nitrobenzenes activated by CF3 or CN groups or by ring-nitrogen with n-butylamine, pyrrolidine, or piperidine. The experiments utilized spectrophotometric kinetic measurements, with the concentration of amine in large excess of the parent concentration, observing first-order kinetics. The study also analyzed the effects of changing the leaving group from chloride to fluoride and to phenoxide, and of the amine from n-butylamine to pyrrolidine and to piperidine on the reaction kinetics. The analyses included evaluating rate constants, examining steric effects, ground-state stabilisation, and base catalysis, with results indicating reduced reactivity with decreasing ring activation and specific steric effects leading to rate-retardation.
10.1002/chem.201301398
The study investigates the cine-substitution reactions of metallabenzenes, focusing on the osmabenzene complex [(SCN)2(PPh3)2Os{CHC(PPh3)CHCICH}] (2). This complex reacts with alcohols like MeOH or EtOH in the presence of strong alkali to yield cine-substitution products with methoxy or ethoxy groups attached. However, when reacted with amines such as n-butylamine or aniline under similar conditions, it produces both the desired cine-substitution products and five-membered ring species. The mechanisms of these reactions are explored through isotopic labeling experiments and DFT calculations, revealing that the reactions proceed via nucleophilic addition, dissociation of the leaving group, protonation, and deprotonation steps, resembling the classical ANRORC mechanism. The study highlights the significant influence of nucleophilicity and basicity on the reaction outcomes, demonstrating that the formation of five-membered ring products is favored kinetically with amines due to their higher nucleophilicity, while the cine-substitution products are more thermodynamically stable.
10.1039/b104419m
The research focuses on the synthesis and characterization of the first organo-tungsten pyrylium salt, (4-cyclopentadienyl-2,6-diphenylpyrylium)W(CO)3CH3, and its transformation into the corresponding pseudobase under aqueous basic conditions. The study aimed to develop a compound that could be used for the labeling of biological molecules and potentially aid in protein structure determination by X-ray crystallography. The tungsten pyrylium complex was synthesized by reacting a deprotonated starting material with a preformed pyrylium salt, followed by hydride abstraction with trityl cation. The complex was characterized using elemental analysis, infrared (IR) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy, which included both proton (1H) and carbon-13 (13C{1H}) analyses. The pseudobase was obtained by treating the tungsten pyrylium salt with Na2CO3 in an acetone-water mixture, followed by extraction with diethyl ether. Its structure was confirmed by 1H NMR analysis and X-ray crystallography, which revealed a four-legged piano stool geometry and significant deviation from planarity in the conjugated chain. The reactivity of the complex with n-butylamine was also studied, showing a pseudo-first order reaction rate. This research could pave the way for new approaches in protein X-ray structural determination.
10.1016/j.tetlet.2013.01.021
The research focuses on the solventless, convenient synthesis of new cyano-2-aminopyridine derivatives from enaminonitriles using microwave irradiation. The purpose of this study was to develop a green approach to synthesize 4-substituted-3-cyano-2-aminopyridines, which are important intermediates in organic synthesis and exhibit various biological properties. The methodology is highlighted for its ease of execution, rapid access, and good yields. The chemicals used in the process include various primary amines such as methylamine, allylamine, butylamine, isopropylamine, and benzylamine, which reacted with enaminonitriles to form the desired 2-aminopyridine derivatives. The study concluded that the solvent-free methods, particularly under microwave activation, were efficient and preferred due to shorter reaction times and higher yields, thus opening a new route for the synthesis of various substituted nitrogen heterocycles.
10.1021/jo00051a017
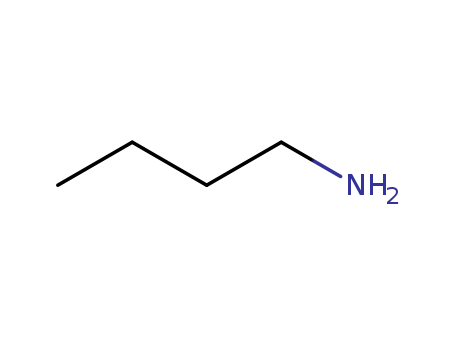
The research investigates the oxidation of various primary amines using dimethyldioxirane (1) and in situ oxidations with oxone. The study explores the formation of different products such as oximes, nitroso dimers, nitroalkanes, nitrones, and oxaziridines under various reaction conditions. Key chemicals involved include cyclohexylamine (2a), n-butylamine (2b), benzylamine (2c), n-decylamine (2d), and 5-methyl-3,4-hexadienylamine (2e). The reactions were performed in solvents like acetone and dichloromethane, with reagents such as NaHCO3 and K2CO3 used as buffering agents. The products were analyzed using techniques like NMR, GC, and MS. The study aims to understand the competing processes and optimize the conditions for specific oxidative transformations of primary amines.


 F,
F, C
C


