Products Categories
| CAS No.: | 77-95-2 |
|---|---|
| Name: | Quinic acid |
| Article Data: | 44 |
| Molecular Structure: | |
|
|
|
| Formula: | C7H12O6 |
| Molecular Weight: | 192.169 |
| Synonyms: | Cyclohexanecarboxylicacid, 1,3,4,5-tetrahydroxy-, (-)- (8CI);Cyclohexanecarboxylic acid, 1,3,4,5-tetrahydroxy-,[1R-(1a,3a,4a,5b)]-;(-)-Quinic acid;D-(-)-Quinic acid;D-Quinic acid;Quinic acid, (-)-; |
| EINECS: | 201-072-8 |
| Density: | 1.828 g/cm3 |
| Melting Point: | 165-170 °C |
| Boiling Point: | 438.4 °C at 760 mmHg |
| Flash Point: | 233.1 °C |
| Solubility: | 400 g/l (20 °C )in water |
| Appearance: | white to light yellow crystal powder |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 36/37/38 |
| Safety: | 22-24/25-36-26 |
| PSA: | 118.22000 |
| LogP: | -2.32140 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile
- 191916-39-9
methyl (-)-quinate

- 77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water for 13h; Ambient temperature; | 100% |
| With water; sodium hydroxide In tetrahydrofuran at 20℃; for 5h; | 90% |
| Multi-step reaction with 4 steps 1: triethylamine / N,N-dimethyl-formamide / 18 h / 0 - 20 °C / Inert atmosphere 2: Dess-Martin periodane / dichloromethane / 20 °C 3: sodium tetrahydroborate / ethanol / 0.67 h / -30 °C 4: hydrogenchloride / water / 1 h View Scheme |
- 135711-58-9
Methyl (1S,3R,4R,5R)-3-<(tert-Butyl)dimethylsilyloxy>-1,4,5-trihydroxycyclohexane-1-carboxylate

- 77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; acetic acid In tetrahydrofuran; water 1) 1 h, 2) 15 h, r.t.; | 90% |
- 202650-88-2
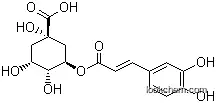
3-caffeoylquinic acid

- 77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With methanol; sodium hydroxide at 60℃; for 4h; | 88% |
| With 3percent HCl at 90℃; for 3h; Product distribution; | |
| Multi-step reaction with 2 steps 1: palladium/charcoal; acetic acid / Hydrogenation 2: aqueous hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| bei der Alkalispaltung; |
- 212891-05-9
1,3-di-O-caffeoylquinic acid

- 77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide | |
| With barium dihydroxide |
- 25514-46-9
(1S,3S,4R,5R)-3-((3-(3,4-dihydroxyphenyl)propanoyl)oxy)-1,4,5-trihydroxycyclohexane-1-carboxylic acid

- 77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| microbial construct AB2848aroD/pKD136/pTW8090A; | 25 mmol |
| With Escherichia coli QP1.1; pKD12138; standard fermentation medium In water at 33 - 37℃; for 48h; pH=7.0; |
- 2450-53-5, 16758-05-7, 89919-61-9, 89919-62-0, 101222-97-3
3,5-dicaffeoylquinic acid

A
- 331-39-5
caffeic acid

B
- 77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With water; unspecific esterase hydrolysis; | |
| With water hydrolysis with unspecific esterase; |
- 114687-85-3
5-O-p-coumaroylquinic acid methyl ester

A
- 7400-08-0
p-Coumaric Acid

B
- 77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 1h; Heating; |

- 77-95-2
D-(-)-quinic acid

| Conditions | Yield |
|---|---|
| With tannase In water at 37℃; for 6h; |
- 814-71-1Acetic acid,2-mercapto-, calcium salt (2:1)
- 7758-05-6Iodic acid, potassium salt
- 506-68-3Cyanogen bromide
- 144245-85-2Alkylketene dimer
- 1332-65-6Copper oxychloride
- 11006-76-1Virginiamycin
- 132-63-8Carbamic acid,N-(7-hydroxy-1-naphthalenyl)-, methyl ester
- 107-06-21,2-Dichloroethane
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Specification
The CAS register number of Quinic acid is 77-95-2. It also can be called as 1,3,4,5-Tetrahydroxycyclohexanecarboxylic acid; D-(-)-Quinic acid and the IUPAC name about this chemical is (3R,5R)-1,3,4,5-tetrahydroxycyclohexane-1-carboxylate. The molecular formula about this chemical is C7H12O6 and the molecular weight is 192.17. It belongs to the following product categories which include Chiral; for Resolution of Bases; Optical Resolution; Synthetic Organic Chemistry; Asymmetric Synthesis; Chiral Resolution Reagents; Chiral Resolving Reagents and so on.
Physical properties about Quinic acid are: (1)# of Rule of 5 Violations: 1; (2)ACD/BCF (pH 5.5): 1; (3)ACD/BCF (pH 7.4): 1; (4)ACD/KOC (pH 5.5): 1; (5)ACD/KOC (pH 7.4): 1; (6)#H bond acceptors: 6; (7)#H bond donors: 5; (8)#Freely Rotating Bonds: 5; (9)Polar Surface Area: 118.22Å2; (10)Index of Refraction: 1.687; (11)Molar Refractivity: 40.04 cm3; (12)Molar Volume: 105 cm3; (13)Polarizability: 15.87x10-24cm3; (14)Surface Tension: 139.5 dyne/cm; (15)Enthalpy of Vaporization: 80.25 kJ/mol; (16)Boiling Point: 438.4 °C at 760 mmHg; (17)Vapour Pressure: 1.58E-09 mmHg at 25°C.
The Quinic acid is an acid which is found in cinchona bark and elsewhere in plants. This chemical is used as an astringent, it is also thought to displace binding of the mu opioid receptor antagonist, however this acid was originally thought to be pharmacologically inactive.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing and do not breathe dust, you also need to keep avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O[C@@H]1C[C@](O)(CC(O)[C@@H]1O)C(O)=O
(2)InChI: InChI=1/C7H12O6/c8-3-1-7(13,6(11)12)2-4(9)5(3)10/h3-5,8-10,13H,1-2H2,(H,11,12)/t3-,4?,5-,7+/m1/s1
(3)InChIKey: AAWZDTNXLSGCEK-RKGSPJAZBM
(4)Std. InChI: InChI=1S/C7H12O6/c8-3-1-7(13,6(11)12)2-4(9)5(3)10/h3-5,8-10,13H,1-2H2,(H,11,12)/t3-,4?,5-,7+/m1/s1
(5)Std. InChIKey: AAWZDTNXLSGCEK-RKGSPJAZSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | 10gm/kg (10000mg/kg) | Zeitschrift fuer die Gesamte Experimentelle Medizin. Vol. 113, Pg. 536, 1944. |