



Products Categories
| CAS No.: | 99-92-3 |
|---|---|
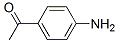
| Name: | 4-Aminoacetophenone |
| Article Data: | 368 |
| Molecular Structure: | |
|
|
|
| Formula: | C8H9NO |
| Molecular Weight: | 135.166 |
| Synonyms: | Acetophenone,4'-amino- (8CI);Acetophenone, p-amino- (3CI);1-(4-Aminophenyl)ethanone;1-Acetyl-4-aminobenzene;4-Acetylaniline;4-Acetylphenylamine;4-Aminophenylmethyl ketone;NSC 3242;p-Acetylaniline;p-Acetylphenylamine;p-Aminoacetophenone;p-Aminoacetylbenzene;p-Aminophenylmethyl ketone;4-Aminoacetophenone; |
| EINECS: | 202-801-2 |
| Density: | 1.096 g/cm3 |
| Melting Point: | 103-107 °C(lit.) |
| Boiling Point: | 294.8 °C at 760 mmHg |
| Flash Point: | 132.1 °C |
| Solubility: | It is soluble in hot water, ethanol and ether. |
| Appearance: | slightly yellow to brown crystalline powder |
| Hazard Symbols: |
 Xn, Xn, Xi Xi
|
| Risk Codes: | 22-36/37/38-20/21/22 |
| Safety: | 26-36 |
| PSA: | 43.09000 |
| LogP: | 2.05260 |

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 20℃; under 760.051 Torr; for 9h; | 100% |
| With hydrogenchloride; ammonium chloride In tetrahydrofuran; water at 45℃; for 2h; Sealed tube; Green chemistry; | 100% |
| With hydrogen In water at 50℃; under 750.075 Torr; for 1.5h; | 100% |
4-(2-methyl-1,3-dithian-2-yl)aniline

4-Aminoacetophenone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; iodine; sodium dodecyl-sulfate In water at 20℃; for 0.333333h; Micellar solution; | 100% |
| With Methylthio(bismethylthio)sulfonium hexachloroantimonate In dichloromethane at -77℃; for 0.0166667h; | 97% |
| With 2,4,4,6-Tetrabromo-2,5-cyclohexadien-1-one; dihydrogen peroxide In water; acetonitrile at 20℃; for 0.5h; | 96% |
| With hydrogen bromide; dihydrogen peroxide In acetonitrile at 25℃; for 6h; | 95% |
4-Ethynylaniline

4-Aminoacetophenone

| Conditions | Yield |
|---|---|
| With gold(III) tribromide; water at 200℃; for 0.333333h; microwave irradiation; | 100% |
| With 5,10,15-tris(pentafluorophenyl)corrole cobalt(III) triphenyl phosphine; sulfuric acid; water In methanol at 80℃; for 12h; | 99% |
| With trifluorormethanesulfonic acid; water In 2,2,2-trifluoroethanol at 25 - 70℃; for 45h; Sealed tube; regioselective reaction; | 89% |
1-(4-(tert-butyldimethylsilylamino)phenyl)ethanone

4-Aminoacetophenone

| Conditions | Yield |
|---|---|
| With silica gel In ethanol; water at 20℃; for 2h; | 100% |
4-azidoacetophenone

4-Aminoacetophenone

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol at 20℃; chemoselective reaction; | 99% |
| With hydrogen; palladium In methanol; N,N-dimethyl-formamide at 20℃; under 6080.41 Torr; for 8h; Solvent; Temperature; Pressure; Inert atmosphere; | 99% |
| With ammonium hydroxide at 90℃; for 0.666667h; | 95% |
4-Iodoacetophenone

4-Aminoacetophenone

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(l) iodide; N,N-dimethylethylenediamine In dimethyl sulfoxide at 130℃; for 5h; Reagent/catalyst; Time; Sealed tube; Inert atmosphere; | 99% |
| Stage #1: 4-Iodoacetophenone With potassium phosphate; 2,2,2-trifluoroacetamide; N,N`-dimethylethylenediamine; copper(l) iodide In N,N-dimethyl-formamide at 45℃; Stage #2: With methanol In N,N-dimethyl-formamide at 45℃; Further stages.; | 98% |
| Stage #1: 4-Iodoacetophenone With copper(l) iodide; D-glucosamine hydrochloride; potassium carbonate In water; acetone at 90℃; for 0.166667h; Stage #2: With ammonia In water; acetone at 90℃; for 28h; | 96% |
1-(4-aminophenyl)ethanol

4-Aminoacetophenone

| Conditions | Yield |
|---|---|
| With silica-supported Jones reagent In dichloromethane for 0.00269444h; | 98.8% |
| With bromopentacarbonylmanganese(I); N-methyl-N,N-di(2-pyridylmethyl)amine; acetone; sodium t-butanolate In toluene at 90℃; for 2h; Inert atmosphere; Schlenk technique; Darkness; | 98% |
| With N-Bromosuccinimide; β‐cyclodextrin In methanol; water; acetone at 20℃; for 10h; | 95% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride for 0.05h; Fries rearrangement; microwave irradiation; | 97% |
| With ytterbium trifluoromethanesulfonate; lithium perchlorate In nitromethane at 100℃; for 8h; Fries rearrangement; | 44% |
| With acetic anhydride; zinc(II) chloride |

| Conditions | Yield |
|---|---|
| With C24H32N2*ClCu; ammonia; potassium carbonate In 1-methyl-pyrrolidin-2-one; methanol at 90℃; under 5171.62 Torr; for 24h; Inert atmosphere; | 97% |
| With copper(ll) sulfate pentahydrate; ammonium hydroxide In PEG1000-DIL; methyl cyclohexane at 60℃; for 4h; | 97% |
| With ammonium hydroxide; potassium phosphate; 1-(5,6,7,8-tetrahydroquinolin-8-yl)-2-methylpropan-1-one; copper(I) bromide In dimethyl sulfoxide at 110℃; for 24h; Inert atmosphere; Sealed tube; | 96% |
tert-butyl 4-acetylphenylcarbamate

4-Aminoacetophenone

| Conditions | Yield |
|---|---|
| With water at 100℃; for 2.5h; | 97% |
| 1. | ipr-mus LD50:381 mg/kg | GEPHDP General Pharmacology. 14 (1983),465. NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . | ||
| 2. | ipr-rat LD50:260 mg/kg | JPETAB Journal of Pharmacology and Experimental Therapeutics. 80 (1944),31. | ||
| 3. | orl-mus LD50:596 mg/kg | GEPHDP General Pharmacology. 14 (1983),465. | ||
| 4. | ipr-mus LD50:300 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . | ||
| 5. | orl-bwd LD50:133 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 12 (1983),355. |
 Xn,Xi
Xn,Xi