Related Products
- CHLORIC ACID
- Chloric acid, calciumsalt, dihydrate (8CI,9CI)
- Chloric acid, sodium salt, mixt. wtih urea
- 7790-94-5Chlorosulfuric acid
- 7790-98-9Perchloricacid,ammoniumsalt
- 7790-99-0Iodine chloride (ICl)
- 7791-03-9Perchloric acid,lithium salt (1:1)
- 7791-07-3Perchloric acid, sodiumsalt, monohydrate (8CI,9CI)
- 7791-08-4Stibine, chlorooxo-
- 7791-11-9Rubidiumchloride (RbCl)
- 7791-12-0Thallium chloride(TlCl)
- 7791-13-1Cobalt chloride(CoCl2), hexahydrate (8CI,9CI)
- 7791-16-4ANTIMONY DICHLOROTRIFLUORIDE
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
CHLORIC ACID |
EINECS | N/A |
| CAS No. | 7790-93-4 | Density | 1.2 g/mL at 25oC |
| PSA | 54.37000 | LogP | 0.27510 |
| Solubility | very soluble H2O [CRC10] | Melting Point |
<-20℃ [CRC10] |
| Formula | ClH O3 | Boiling Point | >100oC |
| Molecular Weight | 84.4591 | Flash Point | N/A |
| Transport Information | N/A | Appearance | N/A |
| Safety | A poison. A strong irritant by ingestion and inhalation. Dangerous fire hazard; ignites organic matter upon contact. A very powerful oxidizing agent. Violent or explosive reaction with oxidizable materials. Aqueous solutions decompose explosively during evaporation. Solutions greater than 40% are unstable. Reacts violently with NH3, Sb, Sb2S3, As2S3, Bi, CuS, PHI4, SnS2, SnS. Reaction with cellulose causes ignition after a delay period. Dangerous reaction with metal sulfides and metal chlorides (e.g., incandescent reaction with antimony trisulfide, arsenic trisulfide, tin(II)sulfide, tin(IV) sulfide, explosion on contact with copper sulfide). Reaction with metals (e.g., antimony, bismuth, iron) forms explosive products. When heated to decomposition it emits toxic fumes of Cl−. See also CHLORATES and CHLORINE. | Risk Codes | R8; R34 |
| Molecular Structure |
|
Hazard Symbols | Toxic by ingestion and inhalation. Strong oxidizer, ignites organic materials on contact. |
| Synonyms |
Chloricacid (HClO3) |
Article Data | 161 |
CHLORIC ACID Synthetic route
- 14989-30-1
hypochloric acid

- 13898-47-0
hypochloric acid

A
- 7647-01-0
hydrogenchloride

B
- 10049-04-4, 25052-55-5
chlorine dioxide

C
- 7732-18-5
water

D
- 7790-93-4
chloric acid
Conditions

| Conditions | Yield |
|---|---|
| In water reaction of HClO2 and HClO in weakly acidic or neutral soln. at ambient temp.;; removing of ClO2 with air;; | A n/a B 97% C n/a D n/a |
| In water reaction of HClO2 and HClO in aq. soln. at ambient temp.; influence of pH;; | |
| In water reaction of HClO2 and HClO in weakly acidic or neutral soln. at ambient temp.; acceleration on low concn. of ClO2(1-); no influence of ClO3(1-);; | |
| In water reaction of HClO2 and HClO in weakly acidic or neutral soln. at ambient temp.;; | |
| In water Kinetics; reaction of HClO2 and HClO in aq. soln. at ambient temp.;; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With hydrogenchloride; platinum In not given byproducts: HCl; reaction by use of a 0.05 n-HCl soln. in presence of colloidal Pt;; | A 0% B n/a C 0% |
Conditions

| Conditions | Yield |
|---|---|
| manganese(ll) chloride In water Kinetics; hydrolysis witout action of light with 0.02 m MnCl2 at 91°C;; | |
| In water Irradiation (UV/VIS); weak diffuse light; first HClO is formed; then HClO3;; | |
| In water hydrolysis without action of light at 91°C: thermochemical data;; |
Conditions

| Conditions | Yield |
|---|---|
| In gas common condensing of educts;; |
- 10049-04-4, 25052-55-5
chlorine dioxide

A
- 7647-01-0
hydrogenchloride

B
- 7601-90-3
perchloric acid

C
- 7790-93-4
chloric acid
Conditions

| Conditions | Yield |
|---|---|
| In water Irradiation (UV/VIS); decompn. in sunlight;; | |
| In water Irradiation (UV/VIS); reaction in sunlight;; | |
| In water Irradiation (UV/VIS); reaction in sunlight;; |
Conditions

| Conditions | Yield |
|---|---|
| With water In not given Electrolysis; | |
| With H2O In not given Electrolysis; |
Conditions

| Conditions | Yield |
|---|---|
| With water; chlorine In water Kinetics; byproducts: CH3CO2H; in buffered soln. (pH at begin 2.11 to 2.35) at 16.-+.1°C;; |
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In water oxidation in fuming HNO3 at 15 - 18°C about 1 month;; | A 0% B n/a |
Conditions

| Conditions | Yield |
|---|---|
| With chlorine In not given 1 mo, at 15-18°C, in concd. HNO3; | |
| With Cl2 In not given 1 mo, at 15-18°C, in concd. HNO3; |
Conditions

| Conditions | Yield |
|---|---|
| With bismuth In water frequently explosion, especially at dissolution of bismuth powder in hot HClO4;; | |
| With bismuth In water frequently explosion;; | |
| With Bi In water frequently explosion, especially at dissolution of bismuth powder in hot HClO4;; |
CHLORIC ACID Chemical Properties
CHLORIC ACID (7790-93-4) 's details.
Molecular Formula:ClHO3
Molecular Weight:84.46
Properties:Colorless solution. Fairly stable in cold H2O up to 30%. Strong oxidant, stable as alkali metal salts. Mp: <20°C, bp: decomp @ 40°C d: 1.282 @ 14.2°C.
Occurrence:Only in aqueous solution.
Molecular Structure:
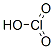
Molecular Formula:ClHO3
Molecular Weight:84.46
Properties:Colorless solution. Fairly stable in cold H2O up to 30%. Strong oxidant, stable as alkali metal salts. Mp: <20°C, bp: decomp @ 40°C d: 1.282 @ 14.2°C.
Occurrence:Only in aqueous solution.
Molecular Structure:
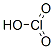
CHLORIC ACID Consensus Reports
Reported in EPA TSCA Inventory.
CHLORIC ACID Safety Profile
A poison. A strong irritant by ingestion and inhalation. Dangerous fire hazard; ignites organic matter upon contact. A very powerful oxidizing agent. Violent or explosive reaction with oxidizable materials. Aqueous solutions decompose explosively during evaporation. Solutions greater than 40% are unstable. Reacts violently with NH3, Sb, Sb2S3, As2S3, Bi, CuS, PHI4, SnS2, SnS. Reaction with cellulose causes ignition after a delay period. Dangerous reaction with metal sulfides and metal chlorides (e.g., incandescent reaction with antimony trisulfide, arsenic trisulfide, tin(II)sulfide, tin(IV) sulfide, explosion on contact with copper sulfide). Reaction with metals (e.g., antimony, bismuth, iron) forms explosive products. When heated to decomposition it emits toxic fumes of Cl−. See also CHLORATES and CHLORINE.
CHLORIC ACID (7790-93-4) has hazard symbols: Toxic by ingestion and inhalation. Strong oxidizer, ignites organic materials on contact.
CHLORIC ACID (7790-93-4) has hazard symbols: Toxic by ingestion and inhalation. Strong oxidizer, ignites organic materials on contact.
CHLORIC ACID Standards and Recommendations
CHLORIC ACID (7790-93-4) 's standards.
DOT Classification: 5.1; Label: Oxidizer
DOT Classification: 5.1; Label: Oxidizer

