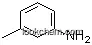
108-44-1Relevant articles and documents
Magnetically nano core–shell Fe3O4@Cu(OH)x: a highly efficient and reusable catalyst for rapid and green reduction of nitro compounds
Shokri, Zahra,Zeynizadeh, Behzad,Hosseini, Seyed Ali,Azizi, Behrooz
, p. 101 - 109 (2017)
Magnetically separable nano core–shell Fe3O4@Cu(OH)x with 22?% Cu content was prepared by the addition of sodium hydroxide to a mixture of CuCl2·2H2O and nano Fe3O4 in water. Characterization of the impregnated copper hydroxide was carried out by X-ray fluorescence (XRF), X-ray diffraction (XRD) atomic absorption spectroscopy (AAS), scanning electron microscopy (SEM), value stream mapping (VSM) and Brunauer–Emmett–Teller (BET) analysis. The core–shell nanocatalyst exhibited the excellent catalytic activity toward reduction of various nitro compounds to the corresponding amines with NaBH4. All reactions were carried out in H2O (55–60?°C) within 3–15?min to afford amines in high to excellent yields. Reusability of core–shell Cu(OH)x catalyst was examined 9?times without significant loss of its catalytic activity.
Arenesulfonic acid-functionalized mesoporous silica decorated with titania: A heterogeneous catalyst for the one-pot photocatalytic synthesis of quinolines from nitroaromatic compounds and alcohols
Hakki, Amer,Dillert, Ralf,Bahnemann, Detlef W.
, p. 565 - 572 (2013)
Acid-modified mesoporous SiO2 decorated with TiO2 (T-S-ArSO3H) was successfully prepared via the co-condensation of 2-(4-chlorosulfonylphenyl)ethyltrimethoxysilane (CSPTMS) and tetraethyl orthosilicate in the presence of commercially available Sachtleben Hombikat UV100 TiO2 particles. The resulting bifunctional catalyst induced the efficient one-pot photocatalytic conversion of nitroaromatic compounds into polyalkylated quinolines in O2-free alcoholic solutions. In this process, a simultaneous reduction of the nitro compound and an oxidation of the alcohol are induced by the photogenerated electrons and holes, respectively. An imine is then produced upon condensation of the generated aldehyde and amino compounds. The cyclization of the produced imine yielding polyalkylated quinoline was found to be catalyzed by the surface attached arene-SO 3H group. The newly synthesized catalyst was characterized by TEM and BET measurements, by FTIR, TGA, as well as by an acid-base titration method.
Simple and Practical Synthesis of Various New Nickel Boride-Based Nanocomposites and their Applications for the Green and Expeditious Reduction of Nitroarenes to Arylamines under Wet-Solvent-Free Mechanochemical Grinding
Mousavi, Hossein,Zeynizadeh, Behzad,Younesi, Reza,Esmati, Mozhgan
, p. 595 - 609 (2018)
In this paper, we report a simple synthesis of four new nickel boride-based nanocomposites, namely Ni2B@ZrCl4, Ni2B@Cu2O, Ni2B@CuCl2 and Ni2B@FeCl3, from commercially available and cheap starting materials. All of the new Ni2B-based nanocomposites were well characterized by Fourier-transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, and energy-dispersive X-ray spectroscopy. Further, the catalytic applications of these new nanocomposites were successfully evaluated in the wet-solvent-free reduction of aromatic nitro compounds to arylamines with sodium borohydride (NaBH4) at room temperature by a mechanochemical grinding technique. All the introduced catalytic systems provide excellent yields of arylamines in very short reaction times for a wide range of substrates. Also, recoverability and reusability of the new nanocomposites were investigated.
Stable and reusable platinum nanocatalyst: An efficient chemoselective reduction of nitroarenes in water
Kotha, Surya Srinivas,Sharma, Nidhi,Sekar, Govindasamy
, p. 1410 - 1413 (2016)
Binaphthyl stabilized Pt nanoparticles (Pt-BNP) have been synthesized, characterized, and utilized as an efficient heterogeneous catalyst for chemoselective reduction of nitroarenes at room temperature in water. Several sensitive functional groups like ketone, ester, acid, amide, halides, and nitrile were well tolerated in this chemoselective reduction. The Pt-BNP catalyst was quantitatively recovered without any major change in particle size and reactivity and then efficiently reused for five catalytic cycles.
Half-sandwich ruthenium complexes with Schiff base ligands bearing a hydroxyl group: Preparation, characterization and catalytic activities
Jia, Wei-Guo,Wang, Zhi-Bao,Zhi, Xue-Ting
, (2020)
Three half-sandwich ruthenium(II) complexes with hydroxyl group functionalized Schiff-base ligands [Ru(p-cymene)LCl] (2a-2c) have been synthesized and characterized. All ruthenium complexes were fully characterized by 1H and 13C NMR spectra, mass spectrometry and infrared spectrometry. The molecular structure of ruthenium complex 2c was confirmed by single-crystal X-ray diffraction methods. Furthermore, these half-sandwich ruthenium complexes were found to exhibit high catalytic activity for nitro compounds reduction using NaBH4 reducing agent in the presence of cetyltrimethylammonium bromide (CTAB) in water at room temperature.
Quinolone-Hydroxyquinoline Tautomerism in Quinolone 3-Esters. Preserving the 4-Oxoquinoline Structure To Retain Antimalarial Activity
Horta, Pedro,Ku, Nihal,Henriques, Marta Sofia C.,Paixo, Jos A.,Coelho, Lis,Nogueira, Ftima,O'Neill, Paul M.,Fausto, Rui,Cristiano, Maria Lurdes Santos
, p. 12244 - 12257 (2015)
Recent publications report in vitro activity of quinolone 3-esters against the bc1 protein complex of Plasmodium falciparum and the parasite. Docking studies performed in silico at the yeast Qo site established a key role for the 4-o
Synthesis, characterization and catalytic activity of gold complexes with pyridine-based selone ligands
Zhang, Hai-Ning,Jia, Wei-Guo,Xu, Qiu-Tong,Ji, Chang-Chun
, p. 315 - 320 (2016)
Three neutral pyridine-based selone compounds, 2,6-bis(1-methylimidazole-2-selone)pyridine (Bmsp), 2,6-bis(1-ethylimidazole-2-selone)pyridine (Besp) and 2,6-bis(1-isopropylimidazole- 2-selone)pyridine (Bpsp) have been synthesized and characterized. Reactions of HAuCl4 with pyridine-based selone ligands result in the formation of the complexes [Au(L)Cl2]+[AuCl2]- (L = Bmsp (1); L = Besp (2) and L = Bpsp (3)), respectively. All compounds have been characterized by elemental analysis, NMR IR spectra and electrospray ionization mass spectroscopic (ESI-MS). The molecular structure of 2 has been determined by X-ray crystallography. Moreover, the gold complexes are efficiently catalyzed nitroarenes reduction to aromatic amines in the presence of sodium tetrahydroborate reducing agent in water.
Reduction of aromatic nitro compounds to amines using zinc and aqueous chelating ethers: Mild and efficient method for zinc activation
Kumar, Pookot Sunil,Lokanatha Rai, Kuriya M.
, p. 772 - 778 (2012)
A mild, environmentally friendly method for reduction of aromatic nitro group to amine is reported, using zinc powder in aqueous solutions of chelating ethers. The donor ether acts as a ligand and also serves as a co-solvent. Water is the proton source. This procedure is also a new method for the activation of zinc for electron transfer reduction of aromatic nitro compounds. The reduction is accomplished in a neutral medium and other reducing groups remained unaffected. The ethers used are dioxolane, 1,4-dioxane, ethoxymethoxyethane, dimethoxymethane, 1,2-dimethoxyethane, and diglyme.
Palladium Immobilized on a Polyimide Covalent Organic Framework: An Efficient and Recyclable Heterogeneous Catalyst for the Suzuki–Miyaura Coupling Reaction and Nitroarene Reduction in Water
Dong, Zhenhua,Pan, Hongguo,Gao, Pengwei,Xiao, Yongmei,Fan, Lulu,Chen, Jing,Wang, Wentao
, p. 299 - 306 (2021/05/10)
An efficient and recyclable Pd nano-catalyst was developed via immobilization of Pd nanoparticles on polyimide linked covalent organic frameworks (PCOFs) that was facilely prepared through condensation of melamine and 3,3′,4,4′-biphenyltetracarboxylic dianhydride. The Pd nanoparticles (Pd NPs) catalyst was thoroughly characterized by FT-IR, XRD, SEM, TEM. Furthermore, the catalytic activity of Pd NPs catalyst was evaluated by Suzuki–Miyaura coupling reaction and nitroarene reduction in water, respectively. The excellent yields of corresponding products revealing revealed that the Pd NPs catalyst could be applied as an efficient and reusable heterogeneous catalyst for above two reactions. Graphical Abstract: [Figure not available: see fulltext.]
Selective Encapsulation and Unusual Stabilization of cis-Isomers by a Spherical Polyaromatic Cavity
Yuasa, Mana,Sumida, Ryuki,Tanaka, Yuya,Yoshizawa, Michito
supporting information, (2022/02/02)
To explore new cavity functions, we herein employed cis-trans stereoisomers with a N=N, C=C, or C=N unit as guest indicators for a polyaromatic capsule. Thanks to the rigid, spherical cavity with a diameter of ~1 nm, azobenzene and stilbene derivatives ar