
Journal of Chemical Thermodynamics p. 1283 - 1296 (1999)
Update date:2022-08-10
Topics:
 Eli, Wumanjiang
Eli, Wumanjiang
 Chen, Wenhai
Chen, Wenhai
 Xue, Qunji
Xue, Qunji
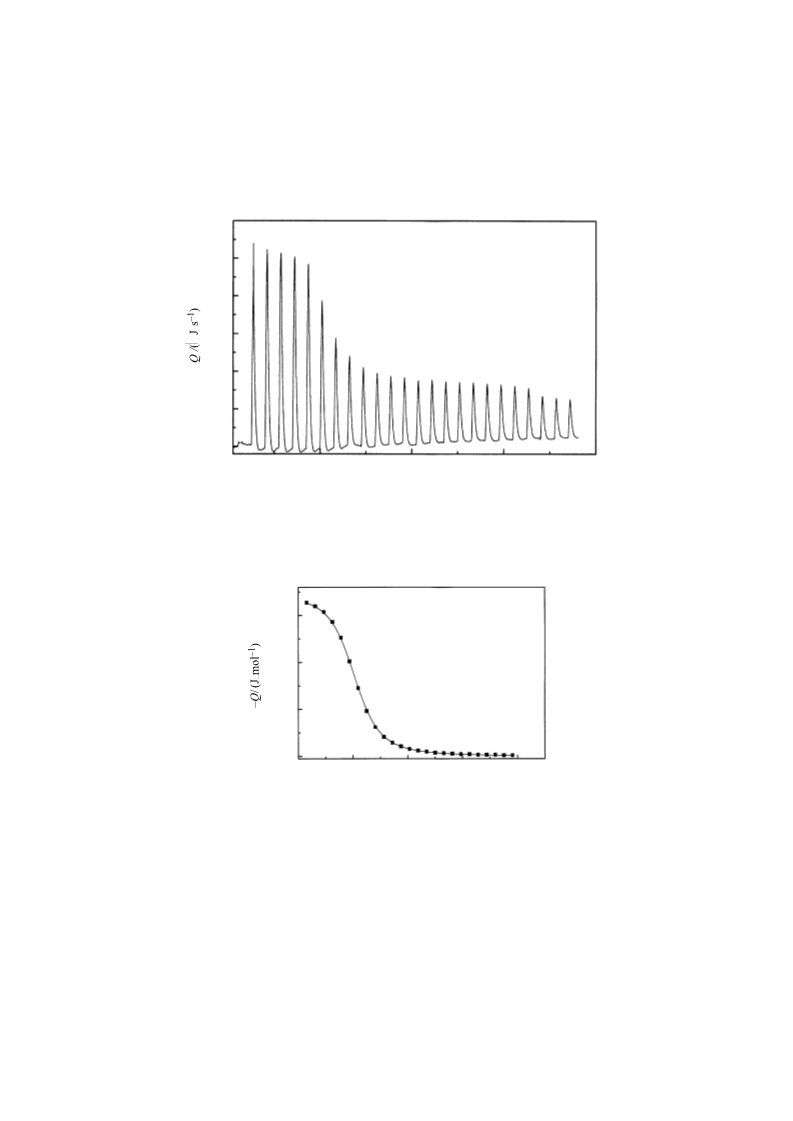
The association of a series of anionic surfactants (CnH2n+1SO4Na, n = 6, 8, 10, 12, 14) with β-cyclodextrin was studied by means of the isothermal titration calorimeter (i.t.c.) at T = 298.15 K. For these types of inclusion complexes, the results agreed well with a 1:1 association mode. Apparent values for the association constants, and changes in the standard molar Gibbs energies, enthalpies, and entropies were derived for the association process. The results indicated that the association of surfactants with β-cyclodextrin is characterized by both favourable enthalpy and favourable entropy changes. The results also demonstrated that the longer the alkyl chain of the anionic surfactant, the greater the association constant with β-cyclodextrin.
View More









Contact:+49-9398-993127
Address:Untertorstr. 27
Shanggao Ruiya Fine Chemicals Co., Ltd
Contact:+86-795-2592103
Address:Xingguang Nanlu,Shanggao County Industry Park
Shanghai Maxchemco Chemical Industry Co., Ltd.
Contact:(86)21-51079223
Address:No.1305-8, B241, the Ecust Park, Huajing Road, Xuhui District, Shanghai
Contact:+49-4101-3053-0
Address:Waldhofstrasse 14 ,25474 Ellerbek Germany
Contact:+86-025-52406782
Address:8 Taizishan Rd., Yanjiang Industrial Development Area, Nanjing, Jiangsu, China.
Doi:10.1021/ja01374a036
(1930)Doi:10.1271/bbb.63.238
(1999)Doi:10.1021/ol991151d
(1999)Doi:10.1016/j.jpcs.2009.11.012
(2010)Doi:10.1039/b605091c
(2006)Doi:10.1016/j.molstruc.2019.127269
(2020)