Article abstract of DOI:10.1006/jcht.1995.0146
The standard (p0 = 0.1 MPa) enthalpies of formation of crystalline dimethylammoniumdimethyldithiocarbamate and of the dimethyldithiocarbamate complexes of Ni(II) and Cu(II) were determined, at the temperature 298.15 K, by solution-reaction calorimetry.The enthalpy of "decomposition" of the dimethylammoniumdimethyldithiocarbamate salt and the enthalpies of sublimation of the metal complexes were measured by high-temperature microcalorimetry.From these values, the mean molar bond-dissociation enthalpies m>(M-S) were derived. .
View More
Full text of DOI:10.1006/jcht.1995.0146
M-3167
J. Chem. Thermodynamics 1995, 27, 1365–1372
Standard enthalpies of formation of crystalline
dimethylammoniumdimethyldithiocarbamate and
of dimethyldithiocarbamate complexes of
copper(II) and nickel(II). The mean Cu–S
and Ni–S bond-dissociation enthalpies
Manuel A. V. Ribeiro da Silva, Ana M. M. V. Reis,
and Rita I. M. C. P. Faria
Centro de Investigacao em Quımica, Department of Chemistry,
Faculty of Science, University of Porto, P-4050 Porto, Portugal
(Received 29 June 1995)
The
standard
(p°
=
0.1 MPa)
enthalpies
of
formation
of
crystalline
dimethylammoniumdimethyldithiocarbamate and of the dimethyldithiocarbamate complexes of
Ni(II) and Cu(II) were determined, at the temperature 298.15 K, by solution-reaction calorimetry.
The enthalpy of ‘‘decomposition’’ of the dimethylammoniumdimethyldithiocarbamate salt and
the enthalpies of sublimation of the metal complexes were measured by high-temperature
microcalorimetry. From these values, the mean molar bond-dissociation enthalpies ꢀDmꢁ(M–S)
were derived.
DfHm°(cr)/(kJ·mol−1
−144.026.4
−146.1210.9
−85.5211.0
)
ꢀDmꢁ(M–S)/(kJ·mol−1
)
(CH3)2NH2S2CN(CH3)2
Ni{S2CN(CH3)2}2
−
22224
18324
Cu{S2CN(CH3)2}2
71995 Academic Press Limited
1. Introduction
The last three decades have witnessed a progressive development of the
thermochemistry of metal dithiocarbamates due to their important applications in
many areas. Most of this thermochemical work has been subject of three major
literature reviews,(1–3) although a critical assessment of the experimental results is
still missing. In previous papers, we reported thermochemical studies of complexes
of copper(II),(4) nickel(II),(5) iron(III),(6) cobalt(III),(6) chromium(III),(6) and
manganese(III),(6) with five different dialkyldithiocarbamates: M(S2CNR2)2 (R=
C2H5, CH3CH2CH2, (CH3)2CH, CH3(CH2)2CH2, and CH3CH(CH3)CH2). In order to
have more thermochemical information on this type of complex and to investigate the
effect of the different alkyl groups in these complexes upon the mean metal–sulfur
molar bond-dissociation enthalpies, ꢀDmꢁ(M–S), thermochemical studies of
0021–9614/95/121365+08 $12.00/0
7 1995 Academic Press Limited
1366
M. A. V. Ribeiro da Silva, A. M. M. V. Reis, and R. I. M. C. P. Faria
dimethylammoniumdimethyldithiocarbamate, and the standard molar enthalpies of
formation and of sublimation of the dimethyldithiocarbamate complexes of copper(II)
and nickel(II) are reported.
2. Experimental
The dimethylammoniumdimethyldithiocarbamate, (CH3)2NH2S2CN(CH3)2, was
prepared as reported by Cavell et al.,(7) by adding to a certain volume of an aqueous
solution containing 40 per cent by volume of dimethylamine (Merck, p.a.) an equal
volume of acetone (Merck, p.a.) followed by the addition of an excess of CS2 (Aldrich
Chemical Co., q99 moles per cent), holding the reaction mixture at a temperature
lower than 283 K, by means of an ice-bath. The solid product was recrystallized from
light petroleum, dried in vacuo, and stored in the dark.
The dimethyldithiocarbamate complexes Ni{S2CN(CH3)2}2 and Cu{S2CN(CH3)2}2,
of nickel(II) and copper(II), were prepared by adding an aqueous solution of the
appropriate AnalaR metal salt: Ni(NO3)2·6H2O or CuSO4·5H2O (50 cm3 containing
0.014 mol), to an aqueous solution containing an excess of (CH3)2NH2S2CN(CH3)2 (in
a mole ratio r=3). The resulting precipitates of metal complexes were filtered, washed
several times with hot water, recrystallized from trichloromethane, and dried over
phosphorus pentoxide.
The purities of the ‘‘ligand’’ and of the complexes were checked by elementary
analyses. The mass-fraction w analyses were as follows:
102·w(C) 102·w(H) 102·w(N) 102·w(C) 102·w(H) 102·w(N)
Expected
Found
(CH3)2NH2S2CN(CH3)2
Cu{S2CN(CH3)2}2
Ni{S2CN(CH3)2}2
36.11
23.71
24.09
8.48
3.98
4.04
16.84
9.22
9.36
36.11
23.75
24.12
8.38
4.01
4.10
16.86
9.25
9.44
From an aqueous solution containing 40 per cent of volume (Merck, p.a.), an
aqueous solution of molality 1 mol·kg−1 was prepared, by dilution with water, and
potentiometrically titrated with a standard solution of HCl(c=0.1 mol·dm−3); the
density of the solution was measured and found to be 0.9839 g·cm−3, leading to the
composition (CH3)2NH·63.39H2O. AnalaR nickel(II) chloride hydrate was powdered
and dried over sodium hydroxide pellets and its composition determined by means of
nickel(II) analyses and found to be NiCl2·6.00H2O; copper sulphate pentahydrate
(Merck, p.a.) was dried over silica gel and its composition was confirmed by means
of copper analyses and found to be CuSO4·5.00H2O; CS2 (Aldrich Chemical Co., q99
moles per cent) and dimethylformamide (Merck, p.a.) were used as supplied.
A glass Dewar calorimeter containing 120 cm3 of solvent, previously described,(8)
was used. The samples were sealed in thin glass ampoules which were broken into the
appropriate solvent by compression between two glass rings. The calorimeter
temperature changes were measured to 10−4 K, at time intervals of 20 s, using a quartz
DfHm°{(CH3)2NH2S2CN(CH3)2,cr} and DfHm°[M{S2CH(CH3)2}2,cr]
1367
thermometer (Hewlett Packard HP 2804A) and recorded by a thermal printer (HP
5150A). The adiabatic temperature change was calculated using the equal-area
method.(9) The calorimeter was calibrated electrically for each experiment. The
accuracy and performance of the calorimeter were tested by measuring the molar
enthalpy of solution of tris(hydroxymethyl)aminomethane, C(CH2OH)3NH2 (THAM,
N.B.S. reference sample 724) in HCl(aq,c=0.100·mol·dm−3) at T=298.15 K. The
value obtained: DsolHm°(cr)=−(29.73620.016) kJ·mol−1 (mean of five experiments)
was in agreement with the literature value of Kilday and Prosen:
−(29.77020.032) kJ·mol−1.(10)
The enthalpy of decomposition of (CH3)2NH2S2CN(CH3)2(cr) and the enthalpies of
sublimation of the Ni(II) and Cu(II) complexes were measured by the ‘‘vacuum
sublimation’’ drop-microcalorimetric method.(11) Samples (of mass about 4 mg) of each
compound, contained in a small thin glass capillary tube sealed at one end, were
dropped, at room temperature, into the hot reaction vessel in the Calvet
High-Temperature Microcalorimeter, held at constant temperature, and then removed
from the hot zone by applying a vacuum. The thermal corrections for the glass capillary
tubes were determined in separate experiments, and were made as small as possible
by dropping tubes of nearly equal mass, to within 210 mg, into the twin calorimeter
cells. The calorimeter was calibrated in situ by making use of the reported enthalpy
of sublimation of napththalene.(12) The quantity measured in the calorimeter:
T
{Hm°(g,T)−Hm°(cr,298.15 K)} was corrected to T=298.15 K, using D298.15 KHm°(g); for
(CH3)2NH2S2CH(CH3)2(cr), the correction term was calculated assuming the gas to be
a mixture of equal amounts of substances of (CH3)2NH and HS2CN(CH3)2. The
T
D298.15 KHm°(g) values for the decomposition and also for the sublimation of the
complexes, were estimated by means of a group-additivity method, as previously
reported,(13) using values reported by Stull et al.(12)
The molar masses used for the elements were those recommended by the IUPAC
Commission;(14) each uncertainty interval quoted is twice the standard deviation of the
mean.
The standard molar enthalpies of formation of the crystalline compound
(CH3)2NH2S2CN(CH3)2 and of the complexes of Ni(II) and Cu(II) were determined by
solution and reaction calorimetry, using the thermochemical reactions:
CS2(l)+2(CH3)2NH·63.39H2O(l)=126.78H2O(l)+
(CH3)2NH2S2CN(CH3)2(cr), (1)
174.00H2O(l)+NiCl2·6H2O(cr)+2(CH3)2NH2S2CN(CH3)2(cr)=
[Ni{S2CN(CH3)2}2](cr)+2(CH3)2NH·63.39H2O(l)+2HCl·26·61H2O(l), (2)
175.32H2O(l)+CuSO4·5H2O(cr)+2(CH3)2NH2S2CN(CH3)2(cr)=
[Cu{S2CN(CH3)2}2](cr)+2(CH3)2NH·63.39H2O(l)+
H2SO4·53.54H2O(l),
(3)
which were carried out in dimethylformamide (120 cm3) as calorimetric solvent. In all
the cases, ampoules containing the reactants were added consecutively, in the order

1368
M. A. V. Ribeiro da Silva, A. M. M. V. Reis, and R. I. M. C. P. Faria
TABLE 1. Molar enthalpies of solution and reaction, at
dimethylammoniumdimethyldithiocarbamate
T = 298.15 K, for
i
Reactant
Solvent
Solution
DiHm/(kJ·mol−1
)
1
2
3
4
CS2(l)
(CH3)2NH·63.39H2O(l)
H2O(l)
DMF
A
DMF
B
A
F
B
F
+3.6620.04
−257.4 21.7
−3.4320.04
+16.1120.12
(CH3)2NH2S2CN(CH3)2(cr)
TABLE 2. Molar enthalpies of solution and reaction, at T=298.15 K, for the nickel(II) complex
i
Reactant
Solvent
Solution
DiHm/(kJ·mol−1
)
1
2
3
4
5
6
H2O(l)
NiCl2·6.00H2O(cr)
(CH3)2NH2S2CN(CH3)2(cr)
Ni{S2CN(CH3)2}2(cr)
(CH3)2NH·63.39H2O(l)
HCl·26.61H2O(l)
DMF
A1
A2
DMF
B1
B2
A1
A2
A3
B1
B2
A3
−3.4520.03
−34.8620.21
+7.1920.24
+15.5320.34
−190.4320.47
−157.7120.75
they are written in the equations, to the solvent, and the corresponding DrHm° values
were measured. To a second portion of the same solvent, were also added
consecutively, ampoules containing the products, and the corresponding DrHm° values
were measured. A rigorous control of the stoichiometry was maintained throughout
each series of experiments to ensure that the final solutions resulting from the
dissolution and reactions were of the same composition as those from the dissolution
and reaction of the products. This was verified by breaking ampoules of the solution
TABLE 3. Molar enthalpies of solution and reaction, at T=298.15 K, for the copper(II) complex
i
Reactant
Solvent
Solution
DiHm/(kJ·mol−1
)
1
2
3
4
5
6
H2O(l)
CuSO4·5.00H2O(l)
(CH3)2NH2S2CN(CH3)2(cr)
Cu{S2CN(CH3)2}2(cr)
(CH3)2NH·63.39H2O(l)
H2SO4·53.54H2O(l)
DMF
A1
A2
DMF
B1
B2
A1
A2
A3
B1
B2
A3
−3.4520.03
+15.1620.58
−13.8020.37
+18.3220.29
−189.4620.96
−281.6220.46
TABLE 4. Standard molar enthalpies of reaction and formation, at T=298.15 K
DrHm°
DfHm°
kJ·mol−1
kJ·mol−1
(CH3)2NH2S2CN(CH3)2(cr)
[Ni{S2CN(CH3)2}2](cr)
[Cu{S2CN(CH3)2}2](cr)
−92.422.4
+60.021.4
+24.921.7
−144.02 6.4
−146.1210.1
−85.5211.0
DfHm°{(CH3)2NH2S2CN(CH3)2,cr} and DfHm°[M{S2CH(CH3)2}2,cr]
TABLE 5. Standard molar enthalpies of formation at T=298.15 K
1369
Compound
DfHm°/(kJ·mol−1
)
H2O(l)
CS2(l)
−285.8320.04 (15)
+89.7 20.7 (15)
(CH3)2NH·63.39H2O(l)
HCl·26.61H2O(l)
H2SO4·53.54H2O(l)
NiCl2·6.00H2O(cr)
CuSO4·5.00H2O(cr)
(CH3)2NH(g)
−70.6 24.2 (16)
−164.4420.01 (15)
−886.8720.30 (15)
−2103.1720.21 (15)
−2279.6520.21 (15)
−18.4620.80 (16)
resulting from the dissolution of the reactants into that resulting from the dissolution
of the products, in the calorimeter, where no enthalpy change was detected.
3. Results
Tables 1 to 3 list the average values of at least five independent determinations of the
molar enthalpies of solution and reaction for the (CH3)2NH2S2CN(CH3)2 compound
and for the nickel(II) and copper(II) complexes, respectively, from which the standard
molar enthalpies of the thermochemical reactions, listed in table 4, were derived by
means of the equations:
DrHm°{(CH3)2NH2S2CN(CH3)2}=D1Hm+2D2Hm−126.78D3Hm−D4Hm,
DrHm°[Ni{S2CN(CH3)2}2]=
(4)
174.00D1Hm+D2Hm+2D3Hm−D4Hm−2D5Hm−2D6Hm, (5)
DrHm°[Cu{S2CN(CH3)2}2]=
175.32D1Hm+D2Hm+2D3Hm−D4Hm−2D5Hm−D6Hm. (6)
The standard molar enthalpies of formation of the crystalline compounds were
derived, with the auxiliary quantities listed in table 5.
The molar enthalpies of ‘‘apparent sublimation’’ of (CH3)2NH2S2CN(CH3)2 are
listed in table 6. Tables 7 and 8 list, respectively, the observed measurements for
TABLE 6. Enthalpy of ‘‘apparent sublimation’’ of a mass m of (CH3)2NH2S2CN(CH3)2
T
T
K
m
mg
D
decHm°(T)
D298.15 KHm°
D
decHm°(298.15 K)
kJ·mol−1
kJ·mol−1
kJ·mol−1
412
412
412
412
412
2.631
3.723
3.532
3.419
2.249
214.4
212.5
214.8
213.4
214.9
24.2
24.2
24.2
24.2
24.2
190.2
188.3
190.6
189.2
190.7
ꢀDdecHm°(298.15 K)ꢁ=(189.820.91) kJ·mol−1

1370
M. A. V. Ribeiro da Silva, A. M. M. V. Reis, and R. I. M. C. P. Faria
TABLE 7. Standard molar enthalpies of sublimation of [Ni{S2CN(CH3)2}2](cr)
g, T
T
g
T
K
Dcr,298.15 KHm
D298.15 KHm°
DcrHm°(298.15 K)
kJ·mol−1
kJ·mol−1
kJ·mol−1
519
519
519
519
519
222.0
222.9
221.4
222.4
221.3
71.6
71.6
71.6
71.6
71.6
150.4
151.3
149.8
150.8
149.7
g
ꢀDcrHm°(298.15 K)ꢁ=(150.420.6) kJ·mol−1
the standard molar enthalpies of sublimation of the complexes of Ni(II) and
Cu(II) and the corrections to T=298.15 K. The standard molar enthalpies of
formation of the complexes in the gaseous state, at T=298.15 K, were derived:
DfHm°[Ni{S2CN(CH3)2}2,g] = (4.3220.9) kJ·mol−1 and DfHm°[Cu{S2CN(CH3)2}2, g]
=(70.5211.0) kJ·mol−1.
4. Discussion
The enthalpy of ‘‘apparent sublimation’’ is the enthalpy of the decomposition reaction
represented by
(CH3)2NH2S2CN(CH3)2(cr)=(CH3)2NH(g)+HS2CN(CH3)2(g),
(7)
from which the value of DfHm°{HS2CN(CH3)2,g}=(64.322.7) kJ·mol−1 was derived.
No experimental values are available for the molar enthalpies of dissociation of the
(S-H) bond in this type of compound. Benson(17) proposed a constant value of
385 kJ·mol−1 for Dm(S-H) in a wide variety of compounds such as H2S, CH3SH,
C2H5SH, and in general Cn H2n+1SH; so, in the absence of an experimental value,
we have used, as in previous papers(4, 5) Dm(S-H) = (38524) kJ·mol−1, to
derive DfHm°{S2CN(CH3)2,g} = (231.3 2 7.5) kJ·mol−1. The mean metal–sulfur
bond-dissociation enthalpies, ꢀDmꢁ(M–S), in M{S2CN(CH3)2}2 complexes may be
TABLE 8. Standard molar enthalpies of sublimation of [Cu{S2CN(CH3)2}2](cr)
g,T
T
g
T
K
Dcr,298 KHm
D298.15 KHm°
DcrHm°(298.15 K)
kJ·mol−1
kJ·mol−1
kJ·mol−1
497
497
497
497
497
219.8
220.3
220.0
220.5
219.7
64.1
64.1
64.1
64.1
64.1
155.7
156.2
155.9
156.4
155.6
g
ꢀDcrHm°(298.15 K)ꢁ=(156.020.3) kJ·mol−1
DfHm°{(CH3)2NH2S2CN(CH3)2,cr} and DfHm°[M{S2CH(CH3)2}2,cr]
1371
defined as one quarter of the standard molar enthalpy of dissociation of the
hypothetical gaseous reaction:
M{S2CN(CH3)2}2(g)=M(g)+2S2CN(CH3)2(g)
(8)
for which
4ꢀDmꢁ(M-S)=DdissHm°=DfHm°(M,g)+2DfHm°{HS2CN(CH3)2,g}−
2DfHm°(H,g)−DfHm°[M{S2CN(CH3)2}2,g]+2Dm(H-S).
(9)
With the auxiliary values: DfHm°(H,g)=(218.0020.01) kJ·mol−1,(18) DfHm°(Ni,g)=
(429.724.2) kJ·mol−1,(15) and DfHm°(Cu,g)=(338.321.2) kJ·mol−1,(15) values of DdissHm°
and
(888
ꢀDmꢁ(M-S)
were
then
and
=
calculated:
ꢀDmꢁ(Ni-S)
(730 2 14) kJ·mol−1 and ꢀDmꢁ(Cu-S)
D
dissHm°[Ni{S2CN(CH3)2}2,g] =
2
14) kJ·mol−1
=
(222 2 4) kJ·mol−1;
D
dissHm°[Cu{S2CN(CH3)2}2,g]
=
(18324) kJ·mol−1.
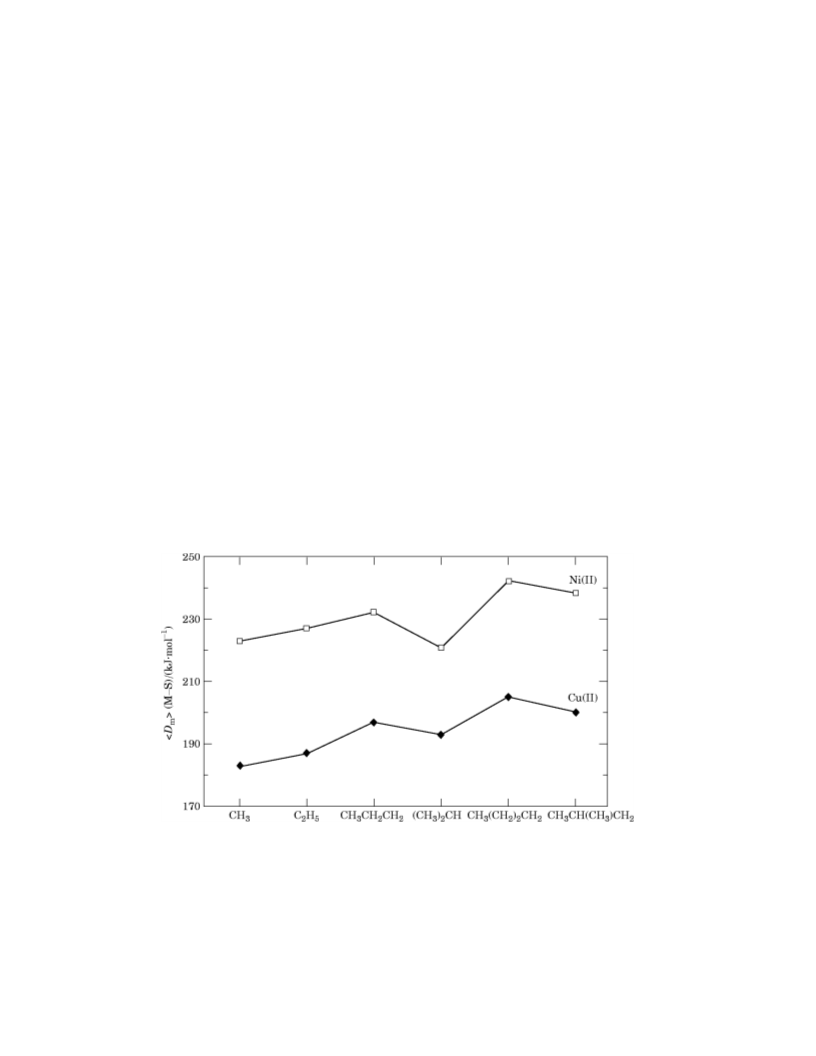
In previous papers(4, 5) we have shown that there is a trend of variation of ꢀDmꢁ(M-S),
M = Cu, Ni with the number of carbon atoms of the alkyl groups of the metal
dithiocarbamates, M{S2CN(CH3)2}2,
R
=
C2H5, CH3CH2CH2, (CH3)2CH,
CH3(CH2)2CH2, and CH3CH(CH3)CH2, and now it is clear that the effect of the CH3
follows the same trend:
Dm(M-S)MeQDm(M-S)EtQDm(M-S)PrQDm(M-S)Bu
as can be seen from figure 1.
FIGURE 1. Variation of ꢀDmꢁ(M-S) for M = Cu(II) or Ni(II) with the alkyl group R in {M(S2CNR2)2}
complexes.
1372
M. A. V. Ribeiro da Silva, A. M. M. V. Reis, and R. I. M. C. P. Faria
Financial support from Instituto Nacional de Investigacao Cientıfica, Lisboa, through
Centro de Investigacao em Quımica da Universidade do Porto (PQ/1-Project–L.5) is
gratefully acknowledged.
REFERENCES
1. Hill, J. O.; Magee, R. J. Rev. Inorg. Chem. 1981, 3, 141.
2. Sharma, A. K. Thermochimica Acta 1986, 104, 339.
3. Hill, J. O.; Murray, R. P. Rev. Inorg. Chem. 1993, 13, 183.
4. Ribeiro da Silva, M. A. V.; Reis, A. M. M. V. J. Chem. Thermodynamics 1989, 21, 167.
5. Ribeiro da Silva, M. A. V.; Reis, A. M. M. V. J. Chem. Thermodynamics 1989, 21, 423.
6. Ribeiro da Silva, M. A. V.; Reis, A. M. M. V. J. Chem. Thermodynamics 1992, 24, 401.
7. Cavell, K. J.; Hill, J. O.; Magee, R. J. J. Inorg. Nucl. Chem. 1979, 41, 1277.
8. Ribeiro da Silva, M. A. V.; Ribeiro da Silva, M. D. M. C.; Dias, A. R. J. Organometallic Chem. 1988,
345, 105.
9. White, W. P. The Modern Calorimeter. The Chemical Catalog Co.: New York. 1928.
10. Kilday, M. V.; Prosen, J. E. J. Res. Nat. Bur. Stand. 1093, A77, 581, 1589.
11. Adedeji, F. A.; Brown, D. L. S.; Connor, J. A.; Leung, M.; Paz-Andrade, M. I.; Skinner, H. A.
J. Organometallic Chem. 1975, 97, 221.
12. Stull, D. R.; Westrum, E. F. Jr.; Sinke, G. C. S. The Chemical Thermodynamics of Organic Compounds.
Wiley: New York. 1969.
13. Ribeiro da Silva, M. A. V.; Reis, A. M. M. V.; Pilcher, G. Thermochimica Acta 1988, 124, 319.
14. J. Phys. Chem. Ref. Data 1993, 22, 6.
15. Wagman, D. D.; Evans, W. H.; Parker, V. B.; Schumm, R. H.; Halow, J.; Bailey, S. M.; Churney,
K. L.; Nuttall, R. J. Phys. Chem. Ref. Data 1982, 11, Suppl. 2.
16. Pedley, J. B.; Naylor, R. D.; Kirby, S. P. Thermochemical Data of Organic Compounds, 2nd edition.
Chapman and Hall: London. 1986.
17. Benson, S. W. Chem. Reviews 1978, 78, 23.
18. J. Chem. Thermodynamics 1978, 10, 903.
Products guided by the article
R&D Labs maybe for 124-40-3
-
Neostar United Industrial Co., Ltd.
Contact:0519-85557386
Address:Yangtze River North Road, Binjiang Economic Development Zone
-
Xi'an Terra Biochem Co. Ltd.
Contact:0086-29-88315623
Address:S711, Innovation Bldg No.25 Gaoxin 1st Rd, Xian P.R of China 710075
-
Wuhan Sunrise Pharmaceutical Technology Co., Ltd
Contact:+86-27-83314682
Address:Room 340, New material Industrial base No.17, Gu Tian Five Lu , Qiaokou District, Wuhan , China
-
Shanghai Synmedia Chemical Co., Ltd
Contact:+86-21-38681880
Address:6th Floor, 11A Building, No.528 Ruiqing Road, Heqing town, Pudong new district, Shanghai China
-
Shanghai TBBMed Co.,Ltd
website:http://www.tbbmed.com
Contact:86--21-50498136
Address:Room 6002, Building 7-1, No.160 Basheng Road,Pudong Area,Shanghai China
Relevant to this article
-
Doi:10.1016/j.tetlet.2015.06.058
(2015)
-
Doi:10.1039/b506245d
(2005)
-
Doi:10.3762/bjoc.9.176
(2013)
-
Doi:10.1021/acs.joc.5b00247
(2015)
-
Doi:10.1002/jhet.5570370441
(2000)
-
Doi:10.1016/j.orgel.2019.105604
(2020)

- ©2008 LookChem.com,License:ICP NO.:Zhejiang16009103 complaints:service@lookchem.com
-
[Hangzhou]86-0571-87562588,87562578,87562573
Our Legal adviser: Lawyer

 Silva, Manuel A. V. Ribeiro da
Silva, Manuel A. V. Ribeiro da
 Reis, Ana M. M. V.
Reis, Ana M. M. V.
 Faria, Rita I. M. C. P.
Faria, Rita I. M. C. P.





