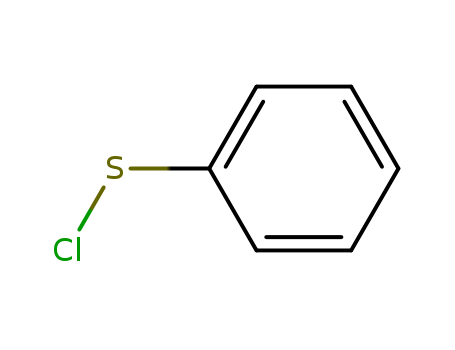10.1016/j.tetlet.2010.03.094
The research focuses on the synthesis and characterization of azidochloromethane and azidobromomethane. These compounds were prepared by treating tris(azidomethyl)amine with dry hydrogen halide. The study explores their role as intermediates in the nucleophilic substitution of dihalomethanes to generate diazidomethane. However, diazidomethane could not be detected in this transformation. The researchers also investigated the reactions of these azides with various reagents, such as cyclooctyne and phenylsulfenyl chloride, leading to the formation of different triazole compounds. The synthesized compounds were characterized using spectroscopic data, and some structures were confirmed by single crystal X-ray diffraction analysis. The research highlights the challenges in handling these hazardous compounds and their potential applications in the chemistry of organic azides.
10.1021/jo00093a024
The research presents an efficient and regio- as well as stereocontrolled methodology for the alkylative bridge cleavage of oxabicyclic vinyl sulfones. The study focuses on a range of 7-oxabicyclo[2.2.1]heptenyl and 8-oxabicyclo[3.2.1]octenyl sulfones, which undergo an overall syn SN2' opening when treated with various organolithium reagents and lithium aluminum hydride. This process yields highly functionalized cyclohexenyl and cycloheptenyl sulfones, which are versatile synthetic intermediates. The chemicals that played crucial roles in this research include organolithium reagents such as methyl lithium (MeLi), n-butyl lithium (n-BuLi), phenyllithium (PhLi), and vinyllithium, as well as lithium aluminum hydride (LAH). Additionally, substrates like oxabicyclic vinyl sulfones, benzyl groups, and phenylsulfonyl groups were essential in the synthesis and transformation processes. The study also involved the use of solvents like tetrahydrofuran (THF) and toluene, and reagents like benzenesulfenyl chloride and methyllithium for the preparation of various vinyl sulfone substrates. The research highlights the importance of these chemicals in achieving the desired regio- and stereocontrolled cleavage of the oxygen bridge in oxabicyclic compounds.
10.1021/ja01606a062
The study investigates the reaction of sulfenyl chlorides with trialkyl phosphites, resulting in the formation of esters of monothiophosphoric acid. Various alkyl and aromatic sulfenyl chlorides, such as methanesulfenyl chloride, benzenesulfenyl chloride, and p-chloroethanesulfenyl chloride, were reacted with triethyl phosphite, tri-n-propyl phosphite, and tri-n-butyl phosphite. The reactions were rapid, even at Dry Ice temperatures, indicating a nucleophilic displacement of chloride accompanied by the elimination of alkyl chloride. The study also compared the reactivity of these sulfenyl chlorides with that of sulfur monochloride and noted that the sulfenyl chlorides reacted at least as readily as acyl halides, which are known to react exothermally with tertiary phosphites. The compounds synthesized were used for biological testing in cancer chemotherapy studies, with particular interest in the 6-chloro thioester as a potential mustard analog.