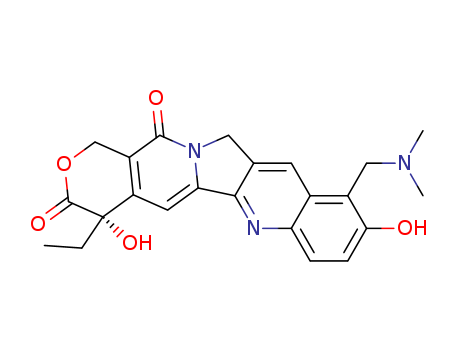
- Chemical Name:Topotecan
- CAS No.:123948-87-8
- Deprecated CAS:133242-28-1,138121-88-7
- Molecular Formula:C23H23N3O5
- Molecular Weight:421.453
- Hs Code.:2942000000
- European Community (EC) Number:687-471-1
- NSC Number:641007
- UNII:7M7YKX2N15
- DSSTox Substance ID:DTXSID3042685
- Nikkaji Number:J2.256.755A,J2.256.757H,J362.027A
- Wikipedia:Topotecan
- Wikidata:Q419953
- NCI Thesaurus Code:C1413
- RXCUI:57308
- Pharos Ligand ID:ND1VZLURMY8N
- Metabolomics Workbench ID:43272
- ChEMBL ID:CHEMBL84
- Mol file:123948-87-8.mol
Synonyms:9 Dimethylaminomethyl 10 hydroxycamptothecin;9-Dimethylaminomethyl-10-hydroxycamptothecin;Hycamtamine;Hycamtin;Hydrochloride, Nogitecan;Hydrochloride, Topotecan;Nogitecan Hydrochloride;NSC 609699;NSC-609699;NSC609699;SK and F 104864 A;SK and F-104864-A;SK and F104864A;SKF 104864 A;SKF-104864-A;SKF104864A;Topotecan;Topotecan Hydrochloride;Topotecan Monohydrochloride, (S)-Isomer


 T,
T, C,
C, F,
F, Xi
Xi


