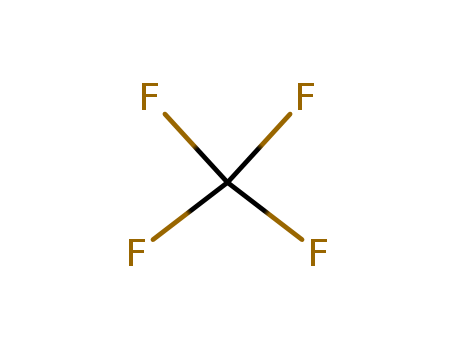
- Chemical Name:Carbon tetrafluoride
- CAS No.:75-73-0
- Deprecated CAS:1519044-53-1,957761-07-8,2209093-32-1
- Molecular Formula:CF4
- Molecular Weight:88.0046
- Hs Code.:
- European Community (EC) Number:200-896-5
- ICSC Number:0575
- UN Number:1982
- UNII:94WG9QG0JN
- DSSTox Substance ID:DTXSID2041757
- Nikkaji Number:J1.464H
- Wikipedia:Tetrafluoromethane,Carbon tetrafluoride,Carbon_tetrafluoride
- Wikidata:Q423055
- RXCUI:1368889
- Metabolomics Workbench ID:56019
- Mol file:75-73-0.mol
Synonyms:carbon tetrafluoride;ftoksilol;ftoxycol;perfluoromethane


 F
F


