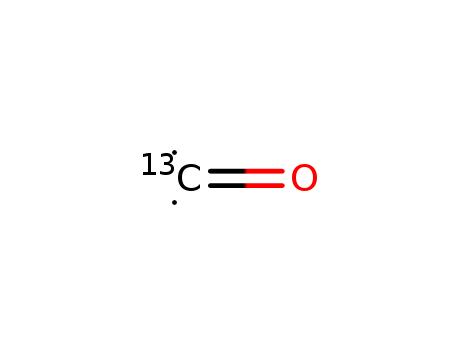- Chemical Name:Carbon monoxide (13C)
- CAS No.:1641-69-6
- Molecular Formula:CO
- Molecular Weight:28.9994
- Hs Code.:
- European Community (EC) Number:683-582-4
- DSSTox Substance ID:DTXSID70430693
- Nikkaji Number:J1.953.777C
- Wikidata:Q82244372
- Mol file:1641-69-6.mol
Synonyms:CARBON MONOXIDE (13C);1641-69-6;Carbon-13C monoxide;(113C)methylidyneoxidanium;DTXSID70430693;Carbon-13C monoxide, <5 atom % 18O, 99 atom % 13C