Products Categories
| CAS No.: | 75-88-7 |
|---|---|
| Name: | 2-Chloro-1,1,1-trifluoroethane |
| Molecular Structure: | |
|
|
|
| Formula: | C2H2 Cl F3 |
| Molecular Weight: | 118.486 |
| Synonyms: | (Chloromethyl)trifluoromethane;1,1,1-Trifluoro-2-chloroethane; 1,1,1-Trifluorochloroethane;1,1,1-Trifluoroethyl chloride; 1-Chloro-2,2,2-trifluoroethane;2,2,2-Trifluoro-1-chloroethane; 2,2,2-Trifluorochloroethane;2,2,2-Trifluoroethyl chloride; 2-Chloro-1,1,1-trifluoroethane; F 133a; FC 133a;Forane 133a; Freon 133a; Genetron 133a; HCFC 133a; R 133a |
| Density: | 1.389 |
| Melting Point: | -105°C |
| Boiling Point: | 7°C |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | R59 |
| Safety: | A poison by inhalation. Experimental reproductive effects. Questionable carcinogen with experimental carcinogenic data. When heated to decomposition it emits toxic fumes of F−. |
| PSA: | 0.00000 |
| LogP: | 1.78750 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; antimony pentafluoride at 100℃; for 1h; Temperature; Autoclave; | 97% |
| With hydrogen fluoride; tantalum pentafluoride at 0 - 140℃; under 25858.1 Torr; for 10.5h; Product distribution / selectivity; Neat (no solvent); | 90% |
| With hydrogen fluoride; tantalum pentafluoride at 100 - 140℃; under 25858.1 Torr; for 6h; | 80% |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; antimonypentachloride at 80 - 90℃; Autoclave; | 92.3% |
| With hydrogen fluoride; antimonypentachloride; chlorine at 120℃; |

| Conditions | Yield |
|---|---|
| With 1,1,2-trichloro-1-fluoroethane; hydrogen fluoride; tantalum pentachloride at 0 - 140℃; for 2h; Product distribution / selectivity; Neat (no solvent); | 87.5% |
| With hydrogen fluoride; tantalum pentachloride at 0 - 140℃; for 2.5h; Product distribution / selectivity; Neat (no solvent); | 22% |
| With antimony dichloride trifluoride; hydrogen fluoride at 144℃; under 33097.9 Torr; |

- 75-89-8
2,2,2-trifluoroethanol


- 421-83-0
trifluoromethane sulfonyl chloride

A

- 75-88-7
1,1,1-trifluoro-2-chloroethane

B

- 333-36-8
1,1,1-trifluoro-2-(2,2,2-trifluoroethoxy)ethane

C

- 6226-25-1
2,2,2-trifluoroethyl trifluoromethanesulphonate

| Conditions | Yield |
|---|---|
| With potassium carbonate; tetra-n-propylammonium bromide In water at 10.2 - 23.3℃; for 4 - 7h; Product distribution / selectivity; | A 0.2 %Chromat. B 0.13 - 0.28 %Chromat. C 82% |


| Conditions | Yield |
|---|---|
| With ammonium persulfate; ammonium formate In N,N-dimethyl-formamide at 30 - 40℃; | 77% |

- 811-95-0
1,1,2-trichloro-1-fluoroethane

A

- 75-88-7
1,1,1-trifluoro-2-chloroethane

B

- 1649-08-7
1,2-dichloro-1,1-difluoroethane

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; tantalum pentachloride at 110℃; for 1.5h; | A 22% B 74% |

- 306-83-2
1,1,1-trifluoro-2,2-dichloroethane

A

- 75-88-7
1,1,1-trifluoro-2-chloroethane

B

- 359-10-4
1-Chloro-2,2-difluoroethene

| Conditions | Yield |
|---|---|
| With water; zinc unter Zusatz eines Netzmittels; |

| Conditions | Yield |
|---|---|
| With aluminum(III) fluoride; hydrogen fluoride at 300℃; | |
| With hydrogen fluoride; boron trifluoride at 25℃; |

- 79-01-6
Trichloroethylene

A

- 75-88-7
1,1,1-trifluoro-2-chloroethane

B

- 354-15-4
1,2,2-trichloro-1,2-difluoroethane

C

- 76-13-1
1,1,2-Trichloro-1,2,2-trifluoroethane

D

- 76-12-0
CFC-112a

| Conditions | Yield |
|---|---|
| With cobalt (III) fluoride at 120℃; Further byproducts given; |


- 79-11-8
chloroacetic acid

A

- 75-88-7
1,1,1-trifluoro-2-chloroethane

B

- 38217-12-8
bis-(2-chloro-1,1-difluoro-ethyl) ether

| Conditions | Yield |
|---|---|
| With sulfur tetrafluoride at 65℃; for 3h; |
- Total:8 Page 1 of 1 1

What can I do for you?
Get Best Price
Chemistry
Molecular Structure of 1,1,1-Trifluoro-2-chloroethane (CAS NO.75-88-7):
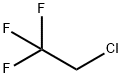
EINECS: 200-912-0
IUPAC Name: 2-Chloro-1,1,1-trifluoroethane
Molecular Formula: C2H2ClF3
Molecular Weight: 118.485490 g/mol
XLogP3-AA: 1.9
H-Bond Donor: 0
H-Bond Acceptor: 3
Canonical SMILES: C(C(F)(F)F)Cl
InChI: InChI=1S/C2H2ClF3/c3-1-2(4,5)6/h1H2
InChIKey: CYXIKYKBLDZZNW-UHFFFAOYSA-N
Index of Refraction: 1.298
Molar Refractivity: 16.62 cm3
Molar Volume: 89.3 cm3
Surface Tension: 14.3 dyne/cm
Density: 1.326 g/cm3
Enthalpy of Vaporization: 24.48 kJ/mol
Boiling Point: 6.9 °C at 760 mmHg
Vapour Pressure: 1430 mmHg at 25 °C
Melting Point: -105 °C
Water Solubility: 365.4 mg/L at 25 °C
BRN: 1733200
Toxicity Data With Reference
| 1. | orl-rat TDLo:78 g/kg/1Y-I:CAR,REP | TXAPA9 Toxicology and Applied Pharmacology. 72 (1984),15. | ||
| 2. | ihl-mus LC50:15 pph/1H | BJANAD British Journal of Anesthesia. 37 (1965),716. |
Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 41 ,1986,p. 253.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory.
Safety Profile
Safety Information of 1,1,1-Trifluoro-2-chloroethane (CAS NO.75-88-7):
Hazard Codes: Xi 
Hazard Note: Irritant
Risk Statements: 59
R59:Dangerous to the ozone layer
Safety Statements: 9-23-59
S9:Keep container in a well-ventilated place
S23:Do not breathe vapour
S59:Refer to manufacturer / supplier for information on recovery / recycling
RIDADR: 1983
TSCA: T
HazardClass: 2.2
A poison by inhalation. Experimental reproductive effects. Questionable carcinogen with experimental carcinogenic data. When heated to decomposition it emits toxic fumes of F−.
Specification
1,1,1-Trifluoro-2-chloroethane with CAS Registry Number of 75-88-7 is a colorless, odorless gas. It is also known as 1,1,1-Trifluoroethyl chloride ; 1-Chloro-2,2,2-trifluoroethane ; 2,2,2-Trifluorochloroethane ; 2-Chloro-1,1,1-trifluoroethane ; 4-01-00-00128 (Beilstein Handbook Reference) ; CCRIS 154 ; CFC 133a ; FC 133a ; Forane 133a ; Freon 133a ; Genetron 133a ; HCFC 133a ; HCFC-133a ; HSDB 6949 ; R 133a ; Chloro-1,1,1-trifluoroethane ; Ethane, 2-chloro-1,1,1-trifluoro- ; 1-Chloro-2,2,2-trifluoroethane or Refrigerant gas R 133a [UN1983] [Non-flammable gas] ; Chlorotrifluoroethane ; HCFC-133a ; UN1983 . It can react violently with strong reducing agents and undergo oxidation with strong oxidizing agents and under extremes of temperature.
