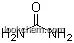Products Categories
| CAS No.: | 7704-34-9 |
|---|---|
| Name: | Sulfur |
| Article Data: | 2983 |
| Molecular Structure: | |
|
|
|
| Formula: | S |
| Molecular Weight: | 33.0739 |
| Synonyms: | Sultaf;Brimstone;Sulfur, solid;sulfur donor;Sublimed sulfur;Super cosan;Kolloidschwefel 95;Sulfidal;Sulfur, rhombic;Ground vocle sulfur;Sulfex;Cosan 80;Sulfur atom;Netzschwefel;Sulforon;Sulfur ointment;Kolofog;Shreesul;Agri-Sul;Thiovit;Sulfur, sublimed;Dispersul;Precipitated sulfur;Ultra Sulfur;Sufran;Sulfur, pharmaceutical;Magnetic 6;Flour sulfur;Spersul thiovit;Kumulus;Gofrativ;Micowetsulf;Kumulus FL;Sulkol;Bensulfoid;Sulsol;Colloidal-S;Sulfur (JP14);Molten Sulfur;Sulfur,Sublimed;sulphur; |
| EINECS: | 231-722-6 |
| Density: | 2.36 g/cm3 |
| Melting Point: | 114 °C |
| Boiling Point: | 445 °C |
| Flash Point: | 168 °C |
| Solubility: | insoluble in water |
| Appearance: | Yellow powder. |
| Hazard Symbols: |
 F F
|
| Risk Codes: | 11 |
| Safety: | 16-26 |
| PSA: | 202.40000 |
| LogP: | 5.18560 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile

| Conditions | Yield |
|---|---|
| at 170-180°C; in very dilute soln. complete decompn. in 2 h, incomplete decompn. in concd. solns.; | A n/a B 100% |
| byproducts: H2S4O6; | |
| sodium thiosulfate In water 100°C; |


| Conditions | Yield |
|---|---|
| With catalyst: CeO2.Co3O4 284-465°C, metal oxide mixture catalysts activity: CuCo2O4; | 100% |
| With catalyst: CuCo2O4 284-465°C, metal oxide mixture catalysts activity: CuCo2O4; | 100% |
| With catalyst: LaCoO3 284-465°C, metal oxide mixture catalysts activity: CuCo2O4; | 100% |

| Conditions | Yield |
|---|---|
| 1690°C complete decompn.; | A 100% B n/a |
| red heat; | A 7% B n/a |
| 400°C; |

| Conditions | Yield |
|---|---|
| With water In water byproducts: H2SO4; 6-8 h at 150-180°C; | A 100% B n/a |
| With H2O In water byproducts: H2SO4; 6-8 h at 150-180°C; | A 100% B n/a |
| With water In water byproducts: H2SO4, SO2; incomplete decompn.; | |
| With H2O In water byproducts: H2SO4, SO2; incomplete decompn.; |

| Conditions | Yield |
|---|---|
| 2000°C, fast react.; | A n/a B 100% |
| 2000°C, fast react.; | A n/a B 100% |
| 1200°C, 2 h; | A n/a B 60% |
| 1200°C, 2 h; | A n/a B 60% |

| Conditions | Yield |
|---|---|
| With water byproducts: H2SO4; 150-180°C under N2; | A 100% B n/a |
| With H2O byproducts: H2SO4; 150-180°C under N2; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| In water Kinetics; Reduction of (NH4)2S2O3 (c=0.4 mole/liter) by H2S in aq. soln. (50°C, pH=5, p(H2S)=0.08 MPa) in presence of Si-based catalyst.; Gravimetrical determination of S.; | A n/a B 99.7% |
| In water Kinetics; Reduction of (NH4)2S2O3 (c=1.0 mole/liter) by H2S in aq. soln. (50°C, pH=5, p(H2S)=0.08 MPa) in presence of Si-based catalyst.; Gravimetrical determination of S.; | A n/a B 99.87% |
| In water Kinetics; Reduction of (NH4)2S2O3 (c=1.0 mole/liter) by H2S in aq. soln. (50°C, pH=5, p(H2S)=0.08 MPa).; Gravimetrical determination of S.; | A n/a B 76.1% |
| In water Kinetics; Reduction of (NH4)2S2O3 (c=0.4 mole/liter) by H2S in aq. soln. (50°C, pH=5, p(H2S)=0.08 MPa).; Gravimetrical determination of S.; | A n/a B 71.5% |


| Conditions | Yield |
|---|---|
| In hydrogenchloride 20°C;satd. solns.; molar ratio 2 : 1; 15 % HCl soln., ,30 min;; S coagulated by addn. of gelatine or Al2(SO4)3; | A n/a B 99.7% |
| In hydrogenchloride 20°C;satd. solns.; molar ratio 2 : 1; 15 % HCl soln., ,30 min;; S coagulated by addn. of gelatine or Al2(SO4)3; | A n/a B 99.7% |
| In hydrogenchloride 20°C; satd. solns.; molar ratio 2 : 1; 3.5 % HCl soln.;; S coagulated by addn. of gelatine or Al2(SO4)3;; | A n/a B 93.5% |


| Conditions | Yield |
|---|---|
| In not given Electrolysis; Pt anode, graphite cathode, area of the electrodes 30 cm^2, 1 A, 20 min, 0.208 mg/l SO2 soln.; | A 98.16% B 70.87% |
| In not given Electrolysis; Pt anode, graphite cathode, area of the electrodes 30 cm^2, 1 A, 20 min, 0.420 mg/l SO2 soln.; | A 98.52% B 74.28% |
| In not given Electrolysis; Pt anode, graphite cathode, area of the electrodes 30 cm^2, 1 A, 20 min, 1.123 mg/l SO2 soln.; | A 98.86% B 74.2% |

| Conditions | Yield |
|---|---|
| In water byproducts: H2O; at ambient temp., molar ratio HS(1-) : HSO3(1-) = 1 : 2 must be adjusted as exactly as possible;; only small amts. of S and sulfate form;; | A n/a B 98% C n/a |
| In water byproducts: H2O; at ambient temp., molar ratio HS(1-) : HSO3(1-) = 1 : 2 must be adjusted as exactly as possible;; only small amts. of S and sulfate form;; | A n/a B 98% C n/a |

 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
History
Sulfur (CAS NO.7704-34-9) was known in ancient times and is referred to in the Torah (Genesis). English translations of the Bible commonly referred to burning sulfur as "brimstone", giving rise to the name of fire-and-brimstone' sermons, in which listeners are reminded of the fate of eternal damnation that await the unbelieving and unrepentant. It is from this part of the Bible that Hell is implied to "smell of sulfur" (likely due to its association with volcanic activity), although sulfur, in itself, is in fact odorless. The "smell of sulfur" usually refers to either the odor of hydrogen sulfide, e.g. from rotten egg, or of burning sulfur, which produces sulfur dioxide, the smell associated with burnt matches. The smell emanating from raw sulfur originates from a slow oxidation in the presence of air. Hydrogen sulfide is the principal odor of untreated sewage and is one of several unpleasant smelling sulfur-containing components of flatulence (along with sulfur-containing mercaptans). A natural form of sulfur known as shiliuhuang was known in China since the 6th century BC and found in Hanzhong. By the 3rd century, the Chinese discovered that sulfur could be extracted from pyrite. Chinese Daoists were interested in sulfur's flammability and its reactivity with certain metals, yet its earliest practical uses were found in traditional Chinese medicine. A Song Dynasty military treatise of 1044 AD described different formulas for Chinese black powder, which is a mixture of potassium nitrate (KNO3), charcoal, and sulfur. Early alchemists gave sulfur its own alchemical symbol which was a triangle at the top of a cross. In 1777, Antoine Lavoisier helped convince the scientific community that sulfur was an element and not a compound. In 1867, sulfur was discovered in underground deposits in Louisiana and Texas. The overlying layer of earth was quicksand, prohibiting ordinary mining operations; therefore, the Frasch process was developed.
Consensus Reports
EPA Extremely Hazardous Substances List. Reported in EPA TSCA Inventory.
Standards and Recommendations
DOT Classification: 4.1; Label: Flammable Solid
Specification
Sulfur (CAS NO.7704-34-9) is insoluble in water and sulfur in its powder is light yellow powder. It is soluble in carbon bisulfide, slightly soluble in ethanol and ethers. Its Synonyms are Sulfur, pharmaceutical ; Sulfur, precipitated [USP] ; Sulfur, sublimed [USP] ; AN-Sulfur Colloid Kit ; Agri-Sul ; Aquilite ; Asulfa-Supra ; Atomic sulfur ; Bensulfoid ; Brimstone .
Physical properties about Sulfur are:
(1)ACD/LogP: 6.117; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 6.12; (4)ACD/LogD (pH 7.4): 6.12; (5)ACD/BCF (pH 5.5): 26242.86; (6)ACD/BCF (pH 7.4): 26242.86; (7)Index of Refraction: 1.924; (8)Molar Refractivity: 65.296 cm3; (9)Molar Volume: 137.756 cm3; (10)Polarizability: 25.886 10-24cm3; (11)Surface Tension: 108.21900177002 dyne/cm; (12)Density: 1.862 g/cm3
Preparation of Sulfur:
Sulfur is produced from petroleum, natural gas, and related fossil resources, from which it is obtained mainly as hydrogen sulfide. Organosulfur compounds, undesirable impurities in petroleum, may be upgraded by subjecting them to hydrodesulfurization, which cleaves the C–S bonds:
R-S-R + 2 H2 → 2 RH + H2S
Uses of Sulfur:
Sulfur is an essential element for all life, and is widely used in biochemical processes. In metabolic reactions, sulfur compounds serve as both fuels and respiratory (oxygen-alternative) materials for simple organisms. Sulfur in organic form is present in the vitamins biotin and thiamine, the latter being named for the Greek word for sulfur. Sulfur is an important part of many enzymes and in antioxidant molecules like glutathione and thioredoxin. Organically bonded sulfur is a component of all proteins, as the amino acids cysteine and methionine. Disulfide bonds are largely responsible for the mechanical strength and insolubility of the protein keratin, found in outer skin, hair, and feathers, and the element contributes to their pungent odor when burned.
Safety information of Sulfur:
When you are using this chemical, please be cautious about it as the following:(1)Keep away from sources of ignition - No smoking; (2)Keep away from combustible material; (3)Handle and open container with care; (4)When using, do not eat or drink; (5)When using, do not smoke; (6)Do not breathe dust; (7)Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer); (8)Avoid contact with skin; (9)Avoid contact with eyes; (10)In case of contact with eyes, rinse immediately with plenty of water and seek medical advice;
You can still convert the following datas into molecular structure:
(1)InChI=1/S ;
(2)Smiles=S;
The toxicity data of Sulfur is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intravenous | 10mg/kg (10mg/kg) | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 29, Pg. 289, 1940. | |
| guinea pig | LDLo | intraperitoneal | 55mg/kg (55mg/kg) | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 29, Pg. 289, 1940. | |
| mammal (species unspecified) | LC50 | inhalation | 1660mg/m3 (1660mg/m3) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 34(12), Pg. 8, 1990. | |
| rabbit | LDLo | intravenous | 5mg/kg (5mg/kg) | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 29, Pg. 289, 1940. | |
| rabbit | LDLo | oral | 175mg/kg (175mg/kg) | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 29, Pg. 289, 1940. | |
| rat | LD | oral | > 8437mg/kg (8437mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 18(5), Pg. 48, 1974. | |
| rat | LDLo | intravenous | 8mg/kg (8mg/kg) | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 29, Pg. 289, 1940. |