Products Categories
| CAS No.: | 92-84-2 |
|---|---|
| Name: | Phenothiazine |
| Article Data: | 124 |
| Molecular Structure: | |
|
|
|
| Formula: | C12H9NS |
| Molecular Weight: | 199.276 |
| Synonyms: | Phenothiazine(6CI,7CI,8CI);Antage TDP;Contaverm;Danikoropa;Dibenzo-1,4-thiazine;Dibenzothiazine;ENT 38;Early bird wormer;Feeno;Fenoverm;NSC 2037;Nemazene;Nexarbol;Orimon;Phenegic;Phenoverm;Phenovis;Phenoxur;Phenzeen;Reconox;Thiodiphenylamine; |
| EINECS: | 202-196-5 |
| Density: | 1.233 g/cm3 |
| Melting Point: | 184 °C |
| Boiling Point: | 371.003 °C at 760 mmHg |
| Flash Point: | 178.176 °C |
| Solubility: | water: 2 mg/L (25 °C) |
| Appearance: | yellow or pale green powder |
| Hazard Symbols: |
 Xi, Xi, N, N, Xn Xn
|
| Risk Codes: | 36/37/38-43-51/53-40-20/21/22 |
| Safety: | 26-61-36/37/39-29-22 |
| PSA: | 37.33000 |
| LogP: | 4.03280 |
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile
- 95789-13-21,3,7-trimethylpurine-2,6-dione
- 956104-42-02-Bromo-5-nitro-3-(trifluoromethyl)pyridine
- 945683-37-41-butyl-2,3-dimethylimidazolium octanesulfate
- 946052-36-41-Butyl-3-methylpyridinium methanesulfonate
- 942196-38-51-Hexyl-3-methylpyridinium hexafluorophosphat
- 929897-32-51-hexyl-4-methylpridine hexafluorophosphate
- 936239-96-24-methyl-1-octylpyridinium tetrafluoroborate(1-)

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,4-dioxane at 18℃; | A n/a B 95% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In ethyl acetate at 20℃; for 2h; | 95% |
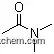
| Stage #1: 10-acetyl-10H-phenothiazine With Triethoxysilane; sodium triethylborohydride In tert-butyl methyl ether at 80℃; for 24h; Stage #2: With hydrogenchloride In tert-butyl methyl ether; water at 20℃; for 1h; chemoselective reaction; | 77% |
| With triethyl borane; sodium hydroxide In tert-butyl methyl ether at 80℃; for 6h; Inert atmosphere; Sealed tube; | 73% |
| Multi-step reaction with 2 steps 1: potassium hydroxide; triethyl borane / tetrahydrofuran / 24 h / 25 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: sodium hydroxide; water / tetrahydrofuran / 1 h / 25 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme |
- 19210-66-3
10-vinyl-10H-phenothiazine

- 92-84-2
10H-phenothiazine

| Conditions | Yield |
|---|---|
| With p-benzoquinone In ethanol at 60℃; for 10h; Rate constant; Thermodynamic data; electron-Affinity Energy; other electron acceptor; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-Bromo-2-iodobenzene; 2-amino-benzenethiol With ferric citrate In N,N-dimethyl-formamide at 80℃; for 2h; Green chemistry; Stage #2: With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 1h; Green chemistry; regioselective reaction; | 94% |
| With copper(l) iodide; potassium carbonate In dimethyl sulfoxide at 120℃; for 48h; Inert atmosphere; regioselective reaction; | 91% |
| With potassium phosphate; copper(l) iodide; N',N'-diphenyl-1H-pyrrole-2-carbohydrazide In diethylene glycol at 90℃; for 7h; Sealed tube; | 70% |
| With N-methoxy-1H-pyrrole-2-carboxamide; copper(II) acetate monohydrate; potassium hydroxide at 90℃; for 15h; Sealed tube; | 64% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran for 24h; Ambient temperature; | A n/a B 92% |
| With n-butyl magnesium bromide In tetrahydrofuran for 20h; Ambient temperature; | A 90% B n/a |
| With n-butyllithium In tetrahydrofuran for 20h; Product distribution; Ambient temperature; Effect of various organometallic reagents (n-BuMgBr, LiTMP, n-BuLi).; | A n/a B 71% |

| Conditions | Yield |
|---|---|
| With isopropyl alcohol In N,N-dimethyl-formamide at 20℃; for 36h; UV-irradiation; chemoselective reaction; | 91% |
| In water; acetonitrile Quantum yield; Photolysis; | 90% |
- 615-42-9
1,2-Diiodobenzene

- 1204-55-3
thioacetic acid S-(2-acetylamino-phenyl)ester

- 92-84-2
10H-phenothiazine

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 130℃; for 10h; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With iodine In acetone for 0.05h; Irradiation; | 90% |
| With iodine; sulfur | 83% |
| With sulfur at 110 - 145℃; Temperature; Inert atmosphere; | 81.1% |

- 92-84-2
10H-phenothiazine

| Conditions | Yield |
|---|---|
| With tetra-n-butylammonium cyanide In acetonitrile at 20℃; for 0.5h; | 90% |
- 72332-01-5
Phenothiazine-10-carboxylic acid 4-diethylamino-butyl ester

A
- 7335-06-0
1-ethylpyrrolidine

B
- 92-84-2
10H-phenothiazine

| Conditions | Yield |
|---|---|
| at 200℃; for 0.5h; Yields of byproduct given; | A n/a B 89% |
- 10405-02-4Trospium chloride
- 2465-27-2Basic Yellow 2
- 87-91-2Butanedioicacid, 2,3-dihydroxy- (2R,3R)-, 1,4-diethyl ester
- 91-22-5Quinoline
- 1185-53-1Tris(hydroxymethyl)aminomethanehydrochloride
- 147536-97-8Bosentan
- 34642-77-8Amoxicillin sodium
- 181289-33-8Hexanoic acid,3-(2-amino-2-oxoethyl)-5-methyl-, (3R)-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Chemistry
IUPAC Name: 10H-Phenothiazine
Synonyms of Phenothiazine (CAS NO.92-84-2): 10H-Phenothiazine ; 4-27-00-01214 (Beilstein Handbook Reference) ; AFI-Tiazin ; AI3-00038 ; Agrazine ; Antiverm ; BRN 0143237 ; Biverm ; CCRIS 5877 ; Caswell No. 652 ; Contaverm ; Dibenzo-1,4-thiazine ; Dibenzo-p-thiazine ; Dibenzoparathiazine ; Dibenzothiazine ; EINECS 202-196-5 ; ENT 38 ; EPA Pesticide Chemical Code 064501 ; Early bird wormer ; Feeno ; Fenothiazine ; Fenothiazine [Dutch] ; Fenotiazina ; Fenotiazina [INN-Spanish] ; Fenotiazina [Italian] ; Fenoverm ; Fentiazin ; HSDB 5279 ; Helmetina ; Lethelmin ; NSC 2037 ; Nemazene ; Nemazine ; Nexarbol ; Orimon ; Padophene ; Reconox ; Souframine ; Thiodifenylamine ; Thiodifenylamine [Dutch] ; Thiodiphenylamin ; Thiodiphenylamin [German] ; Thiodiphenylamine ; Tiodifenilamina ; Tiodifenilamina [Italian] ; UNII-GS9EX7QNU6 ; Vermitin ; Wurm-thional ; XL-50
CAS NO: 92-84-2
Classification Code: Agricultural Chemical ; Anti-Infective Agents ; Antiparasitic Agents ; Antiprotozoal Agents ; Drug / Therapeutic Agent ; Fungicide, bactericide, wood preservative ; Human Data ; Insecticide ; Mutation data ; Nematocide ; Reproductive Effect
Molecular Formula of Phenothiazine (CAS NO.92-84-2): C12H9NS
Molecular Weight: 199.2716
Molecular Structure:
.png)
Melting Point: 184 °C
Polar Surface Area: 37.33 Å2
Index of Refraction: 1.674
Molar Refractivity: 60.69 cm3
Molar Volume: 161.5 cm3
Surface Tension: 52.5 dyne/cm
Density of Phenothiazine (CAS NO.92-84-2): 1.233 g/cm3
Flash Point: 178.2 °C
Enthalpy of Vaporization: 61.8 kJ/mol
Boiling Point: 371 °C at 760 mmHg
Vapour Pressure: 1.06E-05 mmHg at 25°C
Uses
Phenothiazine(CAS NO.92-84-2) is mainly used as the efficient inhibitor for acrylic acid , acrylic esters, and methacrylic acid ester.
There are three main therapeutic applications of the phenothiazine(CAS NO.92-84-2) drugs:
(1) they have an antiemetic effect (stop vomiting);
(2) they are used with anesthetics, potent analgesics (pain relievers), and sedatives to permit their use in smaller doses;
(3) they are used most widely to relieve anx-iety and tension in various severe mental and emotional disorders.
Production
The production of Phenothiazines (CAS NO.92-84-2) involves heating the appropriate meta-substituted diphenylamine with sulfur and an iodine catalyst to close the ring. Treatment with the strong base sodium amide gives the anion on the ring nitrogen, which then displaces the chlorine of the appropriate second reactant.
Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LDLo | unreported | 500mg/kg (500mg/kg) | Journal of the American Chemical Society. Vol. 66, Pg. 888, 1944. | |
| cattle | LD50 | oral | 500mg/kg (500mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 1104, 1986. | |
| child | LDLo | oral | 425mg/kg/5D (425mg/kg) | BEHAVIORAL: HEADACHE LIVER: "JAUNDICE, OTHER OR UNCLASSIFIED" BLOOD: NORMOCYTIC ANEMIA | Lancet. Vol. 242, Pg. 86, 1942. |
| mouse | LD50 | intravenous | 178mg/kg (178mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#00586, | |
| mouse | LD50 | oral | 5gm/kg (5000mg/kg) | BEHAVIORAL: ANTIPSYCHOTIC | Developments in Neuroscience Vol. 7, Pg. 45, 1980. |
| rabbit | LD50 | oral | 4gm/kg (4000mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 1104, 1986. |
Consensus Reports
EPA Genetic Toxicology Program. Reported in EPA TSCA Inventory.
Safety Profile
Poison by intravenous route. Moderately toxic to humans by ingestion. Experimental reproductive effects. An insecticide. Large doses, i.e., heavy exposure, may cause hemolytic anemia and toxic degeneration of the liver. Can cause skin irritation and photosensitization. Dangerous; when heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of SOx and NOx.
Hazard Codes:  Xi,Xn,
Xi,Xn, N
N
Risk Statements: 36/37/38-43-51/53-36/38-40-20/21/22
36/37/38: Irritating to eyes, respiratory system and skin
43: May cause sensitization by skin contact
51/53: Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
36/38: Irritating to eyes and skin
20/21/22: Harmful by inhalation, in contact with skin and if swallowed
Safety Statements: 26-36-61-36/37/39-29-22
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
61: Avoid release to the environment. Refer to special instructions safety data sheet
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
29: Do not empty into drains
22: Do not breathe dust
WGK Germany: 1
F: 8-23: Photosensitive. Sensitive to air.
HS Code: 29343090
Hazardous Substances Data: 92-84-2(Hazardous Substances Data)
Standards and Recommendations
OSHA PEL: TWA 5 mg/m3 (skin)
ACGIH TLV: TWA 5 mg/m3 (skin)
NIOSH REL: (Phenothiazine) TWA 5 mg/m3 (skin)