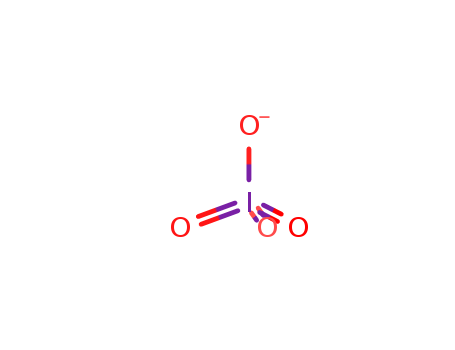
- Chemical Name:Periodate
- CAS No.:15056-35-6
- Molecular Formula:IO4
- Molecular Weight:190.902
- Hs Code.:
- UNII:B45A1BUM4Q
- DSSTox Substance ID:DTXSID80164573
- Nikkaji Number:J301.867I
- Wikipedia:Periodate
- Wikidata:Q27104724
- Mol file:15056-35-6.mol
Synonyms:metaperiodate;NaIO4 compound;periodate;periodate ion;sodium metaperiodate;sodium periodate