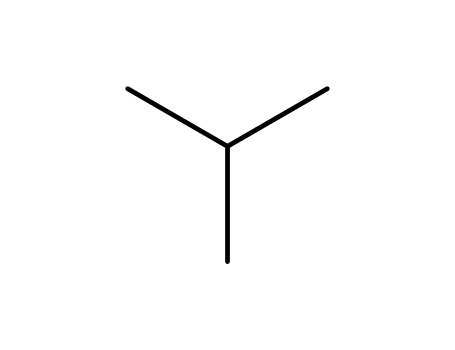
- Chemical Name:Isobutane
- CAS No.:75-28-5
- Deprecated CAS:100494-20-0,70357-15-2,1326305-81-0,70357-15-2
- Molecular Formula:C4H10
- Molecular Weight:58.1234
- Hs Code.:2901100000
- European Community (EC) Number:200-857-2,693-262-6
- ICSC Number:0901
- UN Number:1969,1075
- UNII:BXR49TP611
- DSSTox Substance ID:DTXSID1026401
- Nikkaji Number:J4.159I
- Wikipedia:Isobutane
- Wikidata:Q407225,Q82945424,Q83036171
- NCI Thesaurus Code:C65972
- RXCUI:1363040
- ChEMBL ID:CHEMBL2106398
- Mol file:75-28-5.mol
Synonyms:Butanes;Isobutane;Isobutanes


 F+
F+


