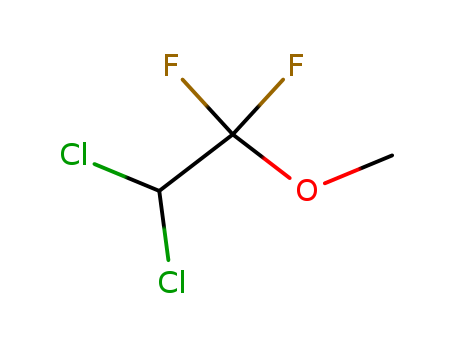
- Chemical Name:Methoxyflurane
- CAS No.:76-38-0
- Deprecated CAS:8056-95-9
- Molecular Formula:C3H4 Cl2 F2 O
- Molecular Weight:164.967
- Hs Code.:2909191800
- European Community (EC) Number:200-956-0
- ICSC Number:1636
- NSC Number:110432
- UN Number:1993
- UNII:30905R8O7B
- DSSTox Substance ID:DTXSID7025556
- Nikkaji Number:J2.405H
- Wikipedia:Methoxyflurane
- Wikidata:Q411594
- NCI Thesaurus Code:C75098
- RXCUI:6857
- Pharos Ligand ID:JPY3G79FHGWZ
- Metabolomics Workbench ID:43270
- ChEMBL ID:CHEMBL1341
- Mol file:76-38-0.mol
Synonyms:Anecotan;Methofluranum;Methoxyflurane;Penthrane;Pentrane