10.1002/hc.21002
The research focuses on the synthesis and 1H NMR titration study of 7-deoxycholic amide or cholane ionophores containing different ion-recognizing groups at C3 and C12. The purpose of this study is to develop new types of ionophores based on a deoxycholic acid backbone (DCAB) that can selectively recognize calcium ions. The researchers synthesized ionophores with a carbamoylpropanamidoacetoxy group at the C3 position and a dithiocarbamoyl group at the C12 position. Key chemicals used in the synthesis include deoxycholic acid, α-chloroacetyl chloride, sodium azide, nanosized zinc powder, and various N,N-di(alkyl, aryl)carbamoylpropanoic acids. The 1H NMR titration experiments showed that the synthesized ionophores form 1:1 complexes with the Ca2+ ion, indicating that the oxygen atoms in the dithiocarbamoyl and carbamoylpropanamidoacetoxy moieties are largely responsible for calcium ion recognition. The conclusion is that these uniquely designed ionophores exhibit high selectivity for calcium ions, which could be useful in the development of ion-selective electrodes and other chemical sensors.


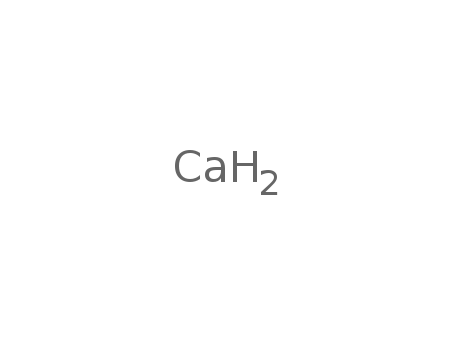
 F
F


