Products Categories
| CAS No.: | 104-88-1 |
|---|---|
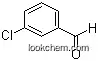
| Name: | 4-Chlorobenzaldehyde |
| Article Data: | 1303 |
| Molecular Structure: | |
|
|
|
| Formula: | C7H5ClO |
| Molecular Weight: | 140.569 |
| Synonyms: | Benzaldehyde, p-chloro-;Benzaldehyde, 4-chloro-;4-chloro benzaldehyde;4-chloroBenzaldehyde;p-chlorobenzenecarboxaldehyde;4-Chlorobenzaldehyde (PCBA);Para-chlorobenzaldehyde;p-Chlorobenzenecarboxaldehyde;p-Chlorobenzaldehyde; |
| EINECS: | 203-247-4 |
| Density: | 1.243 g/cm3 |
| Melting Point: | 46 °C |
| Boiling Point: | 213.713 °C at 760 mmHg |
| Flash Point: | 87.778 °C |
| Solubility: | 935 mg/L (20 °C) in water |
| Appearance: | colourless to light yellow crystalline powder |
| Hazard Symbols: |
 F; F;  C; C;  N; N;  Xn; Xn;  Xi Xi
|
| Risk Codes: | 22-36/37/38-51/53 |
| Safety: | 26-61-37/39-36 |
| Transport Information: | UN 1219 3/PG 2 |
| PSA: | 17.07000 |
| LogP: | 2.15250 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID

| Conditions | Yield |
|---|---|
| With oxygen; perruthenate modified mesoporous silicate MCM-41 In toluene at 80℃; for 1h; Oxidation; | 100% |
| With n-butyltriphenylphosphonium permanganate In acetonitrile at 20℃; for 0.25h; | 100% |
| With butyltriphenylphosphonium chlorochromate In acetonitrile for 0.75h; Heating; | 100% |
- 62102-59-4
2-(4-chlorophenyl)-1,3-benzodithiole

- 104-88-1
4-chlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With tetrafluoroboric acid; mercury(II) oxide In tetrahydrofuran for 0.25h; Ambient temperature; | 100% |

- 104-88-1
4-chlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.0416667h; Product distribution; Ambient temperature; pH = 4-6, regeneration of aldehyde; | 100% |
- 13086-93-6
(acetyloxy)(4-chlorophenyl)methyl acetate

- 104-88-1
4-chlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With [NO(1+)*18-crown-6*H(NO3)2(1-)]; silica gel In dichloromethane at 20℃; for 0.0833333h; | 100% |
| With poly(4-vinylpyridine)-supported sulfuric acid In acetonitrile at 50℃; for 0.75h; Green chemistry; | 100% |
| With bismuth(III) chloride In chloroform for 0.416667h; deprotection; Heating; | 99% |
- 14856-74-7
4-chlorobenzyltrimethylsilyl ether

- 104-88-1
4-chlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With nitrogen dioxide at 20℃; for 0.0833333h; | 100% |
| With trichloroisocyanuric acid In acetonitrile for 0.166667h; Reflux; | 100% |
| With NTPPPODS In acetonitrile for 0.0666667h; Reflux; | 99% |
- 18484-03-2
2-(4-chlorobenzyloxy)tetrahydro-2H-pyran

- 104-88-1
4-chlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With aluminium trichloride; benzyltriphenylphosphonium chlorate In acetonitrile at 20℃; for 1h; | 100% |
| With allyltriphenylphopsphonium peroxodisulfate In acetonitrile for 0.333333h; Heating; | 99% |
| With N-Bromosuccinimide; β‐cyclodextrin In acetone at 20℃; for 0.5h; | 95% |

- 104-88-1
4-chlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With caro's acid; silica gel In dichloromethane for 0.133333h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 4-chlorobenzoate With morpholine; diisobutylaluminium hydride In tetrahydrofuran; hexane at 0℃; for 3.16667h; Inert atmosphere; Stage #2: With diisobutylaluminium hydride In tetrahydrofuran; hexane at 0℃; for 0.166667h; Inert atmosphere; | 99% |
| With phenylsilane; cobalt(II) diacetate tetrahydrate; sodium triethylborohydride In 1,2-dimethoxyethane; toluene at 25℃; for 15h; Inert atmosphere; Schlenk technique; | 84% |
| With n-butyllithium; diisobutylaluminium hydride; tert-butyl alcohol In tetrahydrofuran; hexane at 0℃; | 83% |

| Conditions | Yield |
|---|---|
| With samarium diiodide; phosphoric acid In tetrahydrofuran for 0.000833333h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| With sodium periodate; C53H44As2N2O3Ru In water; ethyl acetate; acetonitrile at 25℃; for 0.5h; | 99% |
| With sodium periodate; C22H23ClIN2Os(1+)*F6P(1-) In water; tert-butyl alcohol at 60℃; for 1h; Schlenk technique; Inert atmosphere; | 99% |
| With sodium periodate; C18H15ClFN2Ru(1+)*Cl(1-) In water; tert-butyl alcohol at 60℃; for 1h; Catalytic behavior; Schlenk technique; Inert atmosphere; | 99% |
- 151096-09-23-Quinolinecarboxylicacid,1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-
- 12150-46-81,1'-Bis(diphenylphosphino)ferrocene
- 26099-09-2Polymaleic acid
- 153439-40-8Benzeneaceticacid, 4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]butyl]-a,a-dimethyl-,hydrochloride (1:1)
- 103-84-4Acetamide,N-phenyl-
- 1582-09-8Trifluralin
- 334-48-5Decanoic acid
- 37830-90-31,3-Dioxol-2-one,4,5-dimethyl-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Consensus Reports
Reported in EPA TSCA Inventory.
Specification
The CAS registry number of p-Chlorobenzaldehyde is 104-88-1. Its EINECS registry number is 203-247-4. The IUPAC name is 4-chlorobenzaldehyde. In addition, the molecular formula is C7H5ClO and the molecular weight is 140.57. It is also called benzaldehyde, 4-chloro-. What's more, it is a kind of colourless to light yellow crystalline powder and belongs to the classes of Fine Chemical & Intermediates; Aromatic Aldehydes & Derivatives (substituted); Benzaldehyde; Aldehydes; C7; Carbonyl Compounds. Besides, it should be stored in sealed container, and put in a cool and dry place. The storage place must stay away from oxidant, the fire and heat source.
Physical properties about this chemical are: (1)ACD/LogP: 2.21; (2)ACD/LogD (pH 5.5): 2.21; (3)ACD/LogD (pH 7.4): 2.21; (4)ACD/BCF (pH 5.5): 28.158; (5)ACD/BCF (pH 7.4): 28.158; (6)ACD/KOC (pH 5.5): 379.525; (7)ACD/KOC (pH 7.4): 379.525; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 1; (10)Polar Surface Area: 17.07 Å2; (11)Index of Refraction: 1.585; (12)Molar Refractivity: 37.901 cm3; (13)Molar Volume: 113.05 cm3; (14)Polarizability: 15.025 ×10-24cm3; (15)Surface Tension: 42.238 dyne/cm; (16)Density: 1.243 g/cm3; (17)Flash Point: 87.778 °C; (18)Enthalpy of Vaporization: 45.003 kJ/mol; (19)Boiling Point: 213.713 °C at 760 mmHg; (20)Vapour Pressure: 0.162 mmHg at 25°C.
Preparation of p-Chlorobenzaldehyde: it can be prepared by 4-chloro-benzaldehyde oxime. This reaction will need reagent poly[N-(4-pyridinumdichromate)-p-styrenesulphonamide] and solvent acetonitrile. The reaction time is 2 hours by heating. The yield is about 90%.

Uses of p-Chlorobenzaldehyde: it can be used as medicine and dye intermediates. And it can react with phenylboronic acid to get biphenyl-4-carbaldehyde. This reaction is a kind of suzuki reaction. It will need reagent K2CO3, catalyst Tedicyp/[PdCl(C3H5)]2 and solvent xylene. The reaction time is 20 hours at reaction temperature of 130 °C. The yield is about 25%.

When you are using this chemical, please be cautious about it as the following:
This chemical is harmful if swallowed. It is irritating to eyes, respiratory system and skin. Moreover, it is toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. When you are using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. In addition, you should avoid release to the environment and you can refer to special instructions safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc(ccc1C=O)Cl
(2)InChI: InChI=1/C7H5ClO/c8-7-3-1-6(5-9)2-4-7/h1-5H
(3)InChIKey: AVPYQKSLYISFPO-UHFFFAOYAQ
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 1400mg/kg (1400mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(2), Pg. 50, 1987. |
| rat | LC | inhalation | > 473mg/m3/4H (473mg/m3) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(2), Pg. 50, 1987. | |
| rat | LD50 | oral | 1575mg/kg (1575mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(2), Pg. 50, 1987. |