Products Categories
| CAS No.: | 63-05-8 |
|---|---|
| Name: | Androstenedione |
| Article Data: | 201 |
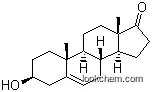
| Molecular Structure: | |
|
|
|
| Formula: | C19H26O2 |
| Molecular Weight: | 286.414 |
| Synonyms: | 17-Ketotestosterone;3,17-Dioxoandrost-4-ene;4-Androstene-3,17-dione;Androstenedione;Fecundin;NSC 9563;SKF 2170;D4-Androstene-3,17-dione;Androstenedione;4-Androstenedione; |
| EINECS: | 200-554-5 |
| Density: | 1.11 g/cm3 |
| Melting Point: | 170-171 °C(lit.) |
| Boiling Point: | 431.4 °C at 760 mmHg |
| Flash Point: | 161.1 °C |
| Solubility: | 57.28mg/L(25 oC) |
| Appearance: | white to off-white solid |
| Hazard Symbols: |
 Xn Xn
|
| Risk Codes: | 40 |
| Safety: | 22-24/25-36 |
| PSA: | 34.14000 |
| LogP: | 4.08740 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE

| Conditions | Yield |
|---|---|
| With Jones reagent In acetone at 0℃; Jones Oxidation; | 100% |
| With oxygen; nitrosonium tetrafluoroborate In dichloromethane at 20℃; for 4h; | 99% |
| With N-chloro-succinimide; dodecyl methyl sulfide; triethylamine In toluene at -40℃; for 16h; Corey-Kim oxidation; | 97% |
- 25375-38-6
3-hydroxy-5-androsten-17-one

- 63-05-8
Androstenedione

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; ferric nitrate In ethyl acetate at 0℃; for 65h; Reagent/catalyst; | 100% |

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 20℃; Electrochemical reaction; | 99% |
| With tetrapropylammonium perruthennate; 4 A molecular sieve; 4-methylmorpholine N-oxide In dichloromethane; acetonitrile for 0.75h; | 95% |
| With chromium(VI) oxide; acetic acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol; water at 40℃; for 2h; pH=3; Temperature; Green chemistry; | 98.6% |
| With sulfuric acid; water; silica gel In toluene for 2h; | 92% |
| Multi-step reaction with 4 steps 1: hydrogenchloride / water; acetone / pH 1-6 2: potassium tert-butylate / tetrahydrofuran / Inert atmosphere; Reflux 3: tetrahydrofuran / Acidic conditions 4: sodium hydrogencarbonate; ruthenium trichloride; Oxone / ethyl acetate; acetonitrile; water / -20 - 30 °C View Scheme |

| Conditions | Yield |
|---|---|
| With calcium hypochlorite; acetic acid In ethyl acetate at 30℃; for 1h; Temperature; Green chemistry; | 98.5% |
- 81176-75-2
(8R,9S,10R,13S,14S,17S)-10,13-dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[α]phenanthrene-3,17-diol

- 63-05-8
Androstenedione

| Conditions | Yield |
|---|---|
| With tetrapropylammonium perruthennate; 4 A molecular sieve; 4-methylmorpholine N-oxide In dichloromethane for 0.5h; | 98% |
| With 9-fluorenone; oxygen In dimethyl sulfoxide at 20℃; Irradiation; | 72% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water Ambient temperature; | 97% |
| With sodium hydroxide In methanol; water at 25℃; | |
| With sodium hydroxide; 3-oxo-Δ5-steroid isomerase In methanol; water Rate constant; pH 5.0 to 5.1; |
- 162657-73-0
3-cycloethylenedithio-17-cycloethylenedioxy-androst-4-ene

- 63-05-8
Androstenedione

| Conditions | Yield |
|---|---|
| With silica gel; copper(II) sulfate In benzene at 80℃; for 5h; | 90% |

| Conditions | Yield |
|---|---|
| aluminum oxide; copper In toluene at 60℃; | A 12% B 88% |

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 20℃; Electrochemical reaction; | 86% |
| With ammonium hydroxide; iodine In water; acetonitrile at 50℃; for 24h; | 78% |
| With manganese(IV) oxide In chloroform for 4h; Heating; | 67.6% |
- 6683-19-8Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate)
- 107-46-0Hexamethyldisiloxane
- 98-79-3L-Pyroglutamic acid
- 2634-33-51,2-Benzisothiazolin-3-one
- 79-81-2Retinol palmitate
- 83919-23-7Mometasone furoate
- 110-87-2Dihydropyran
- 5470-70-2Methyl 6-methylnicotinate
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
History
Andro was legal and able to be purchased over the counter and consequently it was common use in Major League Baseball throughout the 1990s by record-breaking sluggers like Mark McGwire. The supplement is banned by the World Anti-Doping Agency, and hence from the Olympic Games.
On March 12, 2004, the Anabolic Steroid Control Act of 2004 was introduced into the United States Senate. The law took effect on January 20, 2005. Surprisingly, andro was legally defined as an anabolic steroid, even though there is scant evidence that androstenedione itself is anabolic in nature.
On April 11, 2004, the United States Food and Drug Administration banned the sale of Andro.
Androstenedione is currently banned by the US military.
Specification
The Androstenedione is an organic compound with the formula C19H26O2. The IUPAC name of this chemical is (8R,9S,10R,13S,14S)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthrene-3,17-dione. With the CAS registry number 63-05-8, it is also named as 4-Androsten-3,17-dione. The product's categories are Pharmaceutical Intermediates; Steroids; Metabolites & Impurities. Besides, it is a white to off-white solid, which should be stored in a closed place. It is used for biochemical studies.
Physical properties about Androstenedione are: (1)ACD/LogP: 2.90; (2)ACD/LogD (pH 5.5): 2.9; (3)ACD/LogD (pH 7.4): 2.9; (4)ACD/BCF (pH 5.5): 93.64; (5)ACD/BCF (pH 7.4): 93.64; (6)ACD/KOC (pH 5.5): 896.96; (7)ACD/KOC (pH 7.4): 896.96; (8)#H bond acceptors: 2; (9)Polar Surface Area: 34.14 Å2; (10)Index of Refraction: 1.551; (11)Molar Refractivity: 81.71 cm3; (12)Molar Volume: 255.8 cm3; (13)Polarizability: 32.39×10-24cm3; (14)Surface Tension: 42.6 dyne/cm; (15)Density: 1.11 g/cm3; (16)Flash Point: 161.1 °C; (17)Enthalpy of Vaporization: 68.71 kJ/mol; (18)Boiling Point: 431.4 °C at 760 mmHg; (19)Vapour Pressure: 1.2E-07 mmHg at 25°C.
Preparation: this chemical can be prepared by 17-hydroxy-androst-4-en-3-one. This reaction will need reagent CrO3 and acetic acid.

Uses of Androstenedione: it can be used to produce 5,17-dioxo-3,5-seco-A-nor-androstan-3-oic acid. It will need reagent KMnO4 and acetone.

When you are using this chemical, please be cautious about it as the following:
It has limited evidence of a carcinogenic effect. When you are using it, wear suitable protective clothing, do not breathe dust and avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C4/C=C3/CC[C@@H]2[C@H](CC[C@@]1(C(=O)CC[C@H]12)C)[C@@]3(C)CC4
(2)InChI: InChI=1/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1
(3)InChIKey: AEMFNILZOJDQLW-QAGGRKNEBJ
(4)Std. InChI: InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1
(5)Std. InChIKey: AEMFNILZOJDQLW-QAGGRKNESA-N