Products Categories
| CAS No.: | 67-66-3 |
|---|---|
| Name: | Chloroform |
| Article Data: | 654 |
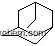
| Molecular Structure: | |
|
|
|
| Formula: | CHCl3 |
| Molecular Weight: | 119.378 |
| Synonyms: | Methyl trichloride;Freon 20;R 20 (refrigerant);Trichloormethaan;Methane trichloride;Cloroformio;Chloroforme;Triclorometano;NCI-C02686;Methenyl trichloride;Methane, trichloro-;Trichlormethan;Trichloroform;Trichloromethane;Methane,trichloro-;Formyl trichloride;Industrial Chloroform;Chloroform, Reagent;Chloroform, Spectrophotometric Grade; |
| EINECS: | 200-663-8 |
| Density: | 1.5 g/cm3 |
| Melting Point: | -63 °C |
| Boiling Point: | 61.2 °C at 760 mmHg |
| Flash Point: | 60.5-61.5°C |
| Solubility: | 8 g/L (20 °C) in water |
| Appearance: | Colorless liquid |
| Hazard Symbols: |
 Xn, Xn,  F, F,  T T
|
| Risk Codes: | 45-46-11-23/24/25-36/37/38-48/20/22-40-38-22-67-66-36/38-41-37/38-39/23/24/25 |
| Safety: | 9-16-26-36-36/37-45-36/37/39-25-23-53-33-7 |
| Transport Information: | UN 1888 6.1/PG 3 |
| PSA: | 0.00000 |
| LogP: | 1.98640 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 75-62-7
Bromotrichloromethane

- 110838-39-6
N,N,N',N'-Tetraisopropyl-P-methylphosphonous diamide

A
- 67-66-3
chloroform

B
- 124862-13-1
P-(bromomethyl)-N,N,N',N'-tetraisopropylphosphonous diamide

| Conditions | Yield |
|---|---|
| In diethyl ether for 0.25h; Ambient temperature; | A 100% B 45% |
| In diethyl ether for 0.25h; Ambient temperature; or P-ethyl-N,N,N',N'-tetraisopropylphosphonous diamide; | A n/a B 45% |

- 75-62-7
Bromotrichloromethane

- 122691-44-5
P-ethyl-N,N,N',N'-tetraisopropylphosphonous diamide

A
- 67-66-3
chloroform

B
- 124862-16-4
P-(1-bromoethyl)-N,N,N',N'-tetraisopropylphosphonous diamide

| Conditions | Yield |
|---|---|
| In diethyl ether for 0.25h; Ambient temperature; | A 100% B 45% |

- 116-16-5
1,1,1,3,3,3-hexachloro-propan-2-one

- 75-31-0
isopropylamine

A
- 67-66-3
chloroform

B
- 23144-67-4
N-Isopropyl-2,2,2-trichloroacetamide

| Conditions | Yield |
|---|---|
| In hexane | A n/a B 100% |

- 1768-31-6
pentachloroacetone

- 75-31-0
isopropylamine

A
- 67-66-3
chloroform

B
- 39063-24-6
N-Isopropyl-2,2-dichloroacetamide

| Conditions | Yield |
|---|---|
| In hexane for 0.5h; | A n/a B 100% |

- 116-16-5
1,1,1,3,3,3-hexachloro-propan-2-one

- 109-73-9
N-butylamine

A
- 67-66-3
chloroform

B
- 31464-96-7
trichloro-acetic acid butylamide

| Conditions | Yield |
|---|---|
| In hexane | A n/a B 100% |

- 1768-31-6
pentachloroacetone

- 74-89-5
methylamine

A
- 67-66-3
chloroform

B
- 5345-73-3
2,2-dichloro-N-methylacetamide

| Conditions | Yield |
|---|---|
| In hexane Heating; | A n/a B 100% |

- 64697-39-8
1,1,1,3,3-pentachlorobutanone

- 74-89-5
methylamine

A
- 67-66-3
chloroform

B
- 83703-95-1
2,2-Dichloro-N-methyl-propionamide

| Conditions | Yield |
|---|---|
| In hexane Heating; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| With tetrachloromethane In toluene under Ar; excess of CCl4 added to suspension of Cp3ZrH in PhMe; stirredat about 20°C for 5 h; stored overnight at 0-5°C; sepd. by pressure; dried in an Ar stream; elem. anal.; | A 99% B 89% |
- 116-16-5
1,1,1,3,3,3-hexachloro-propan-2-one

- 76-37-9
2,2,3,3-tetrafluoropropanol

A
- 67-66-3
chloroform

B
- 1422-70-4
bis(2,2,3,3-tetrafluoropropyl) carbonate

| Conditions | Yield |
|---|---|
| With potassium fluoride; zirconium(IV) oxide at 140℃; for 10h; Product distribution / selectivity; pressure tight reactor; | A 99% B 99% |

 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
History
Chloroform (CAS NO.67-66-3) was discovered in July, 1831 by the American physician Samuel Guthrie,and independently a few months later by the French chemist Eugène Soubeiran and Justus von Liebig in Germany, all of them using variations of the haloform reaction. Soubeiran produced it through the action of chlorine bleach powder on acetone as well as ethanol. It was named and chemically characterised in 1834 by Jean-Baptiste Dumas.
Specification
The Chloroform has the cas register number 67-66-3. This is a kind of colorless liquid, with high refractive index and special sweet odour. Besides it is complete soluble in ethanol, ethyl ether, benzene, petroleum ether, carbon tetrachloride, carbon disulfide and essential oil while slightly soluble in water.
Following are the product information of this chemical: Its product categories are various, including refrigerants; organics; analytical chemistry; solvents for hplc & spectrophotometry; solvents for spectrophotometry; hplc solvents; standard solution of volatile organic compounds for water & soil analysis; standard solutions (voc); method 601cosmetics; 600 series wastewater methods; allergens; alpha sort; chpesticides; fumigantsepa; insecticides; other additives; volatiles/ semivolatiles; auxiliary reagents&products for kf titrationkarl fischer titration reagents (hydranal); karl fischer reagents by application.
The characteristics of this chemical are as follows: (1)ACD/LogP: 1.76; (2)ACD/LogD (pH 5.5): 1.76; (3)ACD/LogD (pH 7.4): 1.76; (4)ACD/BCF (pH 5.5): 12.7; (5)ACD/BCF (pH 7.4): 12.7; (6)ACD/KOC (pH 5.5): 214.66; (7)ACD/KOC (pH 7.4): 214.66; (8)Index of Refraction: 1.445; (9)Molar Refractivity: 21.18 cm3; (10)Molar Volume: 79.5 cm3; (11)Polarizability: 8.39×10-24 cm3; (12)Surface Tension: 28.9 dyne/cm; (13)Density: 1.5 g/cm3; (14)Flash Point: °C; (15)Enthalpy of Vaporization: 29.24 kJ/mol; (16)Boiling Point: 61.2 °C at 760 mmHg; (17)Vapour Pressure: 200 mmHg at 25°C; (18)Exact Mass: 117.914383; (19)MonoIsotopic Mass: 117.914383; (20)Heavy Atom Count: 4; (21)Complexity: 8.
Production method of Chloroform is as below: allylamine could react with 1,1,1,3,3-pentachloro-butan-2-one to produce N-allyl-2,2-dichloro-propionamide and Chloroform, with the following condition: solvent: hexane; reaction time: 30 min; yield: 93.5%.
.jpg)
Use of Chloroform: Chloroform reacts with 1,1-diacetoxy-butane to produce 1,1,1-trichloro-pentan-2-ol, with the following condition: reagent: aq. NaOH; catalytic agent: benzyltriethyammonium chloride ; reaction time: 6 hours; reaction tem.: 40 - 45 ℃; yield: 61%.
.jpg)
As to its usage, it is widely applied in many ways. It could be used in producing Freon22, solvent and anesthetic in pharmaceutics, solvent of rubber, resin, and lipidic; It could be used in organic synthesis material, and also as solvent and extractant; in antibiotic, spice, resin, and so on; It could mix with carbon tetrachloride to produce ice-free fireproofing liquid and also in gun propellant of smolke agent, fumigant of frumentaceous, and standard liquid of base measuring temperature.
While dealing with this chemical, you should be very cautious. For one thing, this chemical is irritant to eyes, respiratory system and skin; For another thing, it is harmful to our health. It could have danger of serious damage to health by prolonged exposure through inhalation and if swallowed, and repeated exposure may cause skin dryness or cracking. Then, it is toxic and it has the danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed; It may cause cancer and heritable genetic damage. Lastly, it is highly flammable: It may catch fire in contact with air, only needing brief contact with an ignition source; It has a very low flash point or evolve highly flammable gases in contact with water; Vapours may cause drowsiness and dizziness
Therefore, you should take the following instructions. Firstly, wear suitable protective clothing, gloves and eye/face protection and keep away from sources of ignition - No smoking. In case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). Secondly, be carful while keeping. Keep container tightly closed and in a well-ventilated place. Thirdly, do not breathe gas/fumes/vapour/spray (appropriate wording to be specified by the manufacturer), and avoid exposure - obtain special instructions before use; Besides. take precautionary measures against static discharges.
Additionally, the following datas could be converted into the molecular structure:
(1)SMILES:ClC(Cl)Cl
(2)InChI:InChI=1/CHCl3/c2-1(3)4/h1H
(3)InChIKey:HEDRZPFGACZZDS-UHFFFAOYAG
Below are the toxicity informatin of this chemical:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LCLo | inhalation | 35gm/m3/4H (35000mg/m3) | BEHAVIORAL: GENERAL ANESTHETIC GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS GASTROINTESTINAL: NAUSEA OR VOMITING | Archiv fuer Hygiene und Bakteriologie. Vol. 116, Pg. 131, 1936. |
| dog | LCLo | inhalation | 100gm/m3 (100000mg/m3) | Pesticide Chemicals Official Compendium, Association of the American Pesticide Control Officials, Inc., 1966. Vol. -, Pg. 230, 1966. | |
| dog | LD50 | intraperitoneal | 1gm/kg (1000mg/kg) | LIVER: LIVER FUNCTION TESTS IMPAIRED | Toxicology and Applied Pharmacology. Vol. 10, Pg. 119, 1967. |
| dog | LDLo | intravenous | 75mg/kg (75mg/kg) | Quarterly Journal of Pharmacy & Pharmacology. Vol. 7, Pg. 205, 1934. | |
| dog | LDLo | oral | 1gm/kg (1000mg/kg) | Quarterly Journal of Pharmacy & Pharmacology. Vol. 7, Pg. 205, 1934. | |
| frog | LCLo | inhalation | 6gm/m3 (6000mg/m3) | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 97, Pg. 86, 1923. | |
| guinea pig | LCLo | inhalation | 20000ppm/2H (20000ppm) | Fluorine Chemistry Reviews. Vol. 1, Pg. 197, 1967. | |
| guinea pig | LD50 | oral | 820mg/kg (820mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 48(3), Pg. 10, 1983. | |
| human | LCLo | inhalation | 25000ppm/5M (25000ppm) | Tabulae Biologicae. Vol. 3, Pg. 231, 1933. | |
| human | TCLo | inhalation | 10mg/m3/1Y (10mg/m3) | BEHAVIORAL: ANOREXIA (HUMAN GASTROINTESTINAL: NAUSEA OR VOMITING GASTROINTESTINAL: OTHER CHANGES | Internationales Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 24, Pg. 127, 1967. |
| human | TCLo | inhalation | 5000mg/m3/7M (5000mg/m3) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" | Archiv fuer Hygiene und Bakteriologie. Vol. 116, Pg. 131, 1936. |
| mammal (species unspecified) | LCLo | inhalation | 25000ppm/5M (25000ppm) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 138, Pg. 65, 1928. | |
| man | LDLo | oral | 2514mg/kg (2514mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) CARDIAC: OTHER CHANGES KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | American Journal of Emergency Medicine. Vol. 6, Pg. 507, 1988. |
| man | LDLo | unreported | 546mg/kg (546mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| mouse | LCLo | inhalation | 23gm/m3/56M (23000mg/m3) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Biochemische Zeitschrift. Vol. 115, Pg. 235, 1921. |
| mouse | LD50 | intraperitoneal | 623mg/kg (623mg/kg) | Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 18, Pg. 109, 1960. | |
| mouse | LD50 | oral | 36mg/kg (36mg/kg) | Archives of Toxicology, Supplement. Vol. 2, Pg. 371, 1979. | |
| mouse | LD50 | subcutaneous | 704mg/kg (704mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 123, Pg. 224, 1958. | |
| rabbit | LCLo | inhalation | 59gm/m3 (59000mg/m3) | Pesticide Chemicals Official Compendium, Association of the American Pesticide Control Officials, Inc., 1966. Vol. -, Pg. 230, 1966. | |
| rabbit | LD50 | skin | > 20gm/kg (20000mg/kg) | National Technical Information Service. Vol. AD-A062-138, | |
| rabbit | LDLo | oral | 500mg/kg (500mg/kg) | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 97, Pg. 86, 1923. | |
| rabbit | LDLo | subcutaneous | 800mg/kg (800mg/kg) | Quarterly Journal of Pharmacy & Pharmacology. Vol. 7, Pg. 205, 1934. | |
| rat | LC50 | inhalation | 47702mg/m3/4H (47702mg/m3) | Environmental Research. Vol. 40, Pg. 411, 1986. | |
| rat | LD50 | intraperitoneal | 894mg/kg (894mg/kg) | Environmental Research. Vol. 40, Pg. 411, 1986. | |
| rat | LD50 | oral | 695mg/kg (695mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Khigiena i Zdraveopazvane. Hygiene and Sanitation. Vol. 29(5), Pg. 39, 1986. |