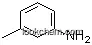
108-44-1Relevant articles and documents
Magnetically nano core–shell Fe3O4@Cu(OH)x: a highly efficient and reusable catalyst for rapid and green reduction of nitro compounds
Shokri, Zahra,Zeynizadeh, Behzad,Hosseini, Seyed Ali,Azizi, Behrooz
, p. 101 - 109 (2017)
Magnetically separable nano core–shell Fe3O4@Cu(OH)x with 22?% Cu content was prepared by the addition of sodium hydroxide to a mixture of CuCl2·2H2O and nano Fe3O4 in water. Characterization of the impregnated copper hydroxide was carried out by X-ray fluorescence (XRF), X-ray diffraction (XRD) atomic absorption spectroscopy (AAS), scanning electron microscopy (SEM), value stream mapping (VSM) and Brunauer–Emmett–Teller (BET) analysis. The core–shell nanocatalyst exhibited the excellent catalytic activity toward reduction of various nitro compounds to the corresponding amines with NaBH4. All reactions were carried out in H2O (55–60?°C) within 3–15?min to afford amines in high to excellent yields. Reusability of core–shell Cu(OH)x catalyst was examined 9?times without significant loss of its catalytic activity.
Studies on carcinogenic azo dyes. I. Synthesis of azo dyes labeled with tritium at the specific position
Baba,Mori,Iwao,Iwahara
, p. 1158 - 1162 (1969)
-
-
Strand,Kovacic
, p. 2977,2981 (1973)
-
-
Kovacic et al.
, p. 1262,1265 (1965)
-
Half-sandwich ruthenium complexes with Schiff base ligands bearing a hydroxyl group: Preparation, characterization and catalytic activities
Jia, Wei-Guo,Wang, Zhi-Bao,Zhi, Xue-Ting
, (2020)
Three half-sandwich ruthenium(II) complexes with hydroxyl group functionalized Schiff-base ligands [Ru(p-cymene)LCl] (2a-2c) have been synthesized and characterized. All ruthenium complexes were fully characterized by 1H and 13C NMR spectra, mass spectrometry and infrared spectrometry. The molecular structure of ruthenium complex 2c was confirmed by single-crystal X-ray diffraction methods. Furthermore, these half-sandwich ruthenium complexes were found to exhibit high catalytic activity for nitro compounds reduction using NaBH4 reducing agent in the presence of cetyltrimethylammonium bromide (CTAB) in water at room temperature.
Synthesis, characterization and catalytic activity of gold complexes with pyridine-based selone ligands
Zhang, Hai-Ning,Jia, Wei-Guo,Xu, Qiu-Tong,Ji, Chang-Chun
, p. 315 - 320 (2016)
Three neutral pyridine-based selone compounds, 2,6-bis(1-methylimidazole-2-selone)pyridine (Bmsp), 2,6-bis(1-ethylimidazole-2-selone)pyridine (Besp) and 2,6-bis(1-isopropylimidazole- 2-selone)pyridine (Bpsp) have been synthesized and characterized. Reactions of HAuCl4 with pyridine-based selone ligands result in the formation of the complexes [Au(L)Cl2]+[AuCl2]- (L = Bmsp (1); L = Besp (2) and L = Bpsp (3)), respectively. All compounds have been characterized by elemental analysis, NMR IR spectra and electrospray ionization mass spectroscopic (ESI-MS). The molecular structure of 2 has been determined by X-ray crystallography. Moreover, the gold complexes are efficiently catalyzed nitroarenes reduction to aromatic amines in the presence of sodium tetrahydroborate reducing agent in water.
Palladium Immobilized on a Polyimide Covalent Organic Framework: An Efficient and Recyclable Heterogeneous Catalyst for the Suzuki–Miyaura Coupling Reaction and Nitroarene Reduction in Water
Dong, Zhenhua,Pan, Hongguo,Gao, Pengwei,Xiao, Yongmei,Fan, Lulu,Chen, Jing,Wang, Wentao
, p. 299 - 306 (2021/05/10)
An efficient and recyclable Pd nano-catalyst was developed via immobilization of Pd nanoparticles on polyimide linked covalent organic frameworks (PCOFs) that was facilely prepared through condensation of melamine and 3,3′,4,4′-biphenyltetracarboxylic dianhydride. The Pd nanoparticles (Pd NPs) catalyst was thoroughly characterized by FT-IR, XRD, SEM, TEM. Furthermore, the catalytic activity of Pd NPs catalyst was evaluated by Suzuki–Miyaura coupling reaction and nitroarene reduction in water, respectively. The excellent yields of corresponding products revealing revealed that the Pd NPs catalyst could be applied as an efficient and reusable heterogeneous catalyst for above two reactions. Graphical Abstract: [Figure not available: see fulltext.]
Rhodium nanoparticles supported on 2-(aminomethyl)phenols-modified Fe3O4 spheres as a magnetically recoverable catalyst for reduction of nitroarenes and the degradation of dyes in water
Chen, Tian,Chen, Zhangpei,Hu, Jianshe,Lv, Kexin,Reheman, Aikebaier,Wang, Gongshu
, (2021/06/18)
A magnetic nanostructured catalyst (Fe3O4@SiO2-Amp-Rh) modified with 2-(aminomethyl)phenols (Amp) was designed and prepared, which is used to catalyze the reduction of aromatic nitro compounds into corresponding amines and the degradation of dyes. The 2-aminomethylphenol motif plays a vital role in the immobilization of rhodium nanoparticles to offer extraordinary stability, which has been characterized by using various techniques, including transmission electron microscopy (TEM), thermal gravimetric analyzer (TGA), X-Ray Diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). A variety of nitroaromatic derivatives have been reduced to the corresponding anilines in water with up to yields of 99% within 1?h at room temperature. In addition, the catalyst system is effective in catalyzing the reduction of toxic pollutant 4-nitrophenol and the degradation of MO, MB and RhB dyes. Importantly, this catalyst Fe3O4@SiO2-Amp-Rh can be easily recovered by an external magnetic field because of the presence of magnetic core of Fe3O4, and the activity of Fe3O4@SiO2-Amp-Rh does not decrease significantly after 7 times’ recycling, which indicates that the catalyst performed high reactivity as well as stability. Graphical abstract: [Figure not available: see fulltext.]
Highly efficient hydrogenation reduction of aromatic nitro compounds using MOF derivative Co-N/C catalyst
Dai, Yuyu,Li, Xiaoqing,Wang, Likai,Xu, Xiangsheng
, p. 22908 - 22914 (2021/12/24)
The direct hydrogenation reduction of aromatic nitro compounds to aromatic amines with non-noble metals is an attractive area. Herein, the pyrolysis of Co(2-methylimidazole)2 metal-organic framework successfully produces a magnetic Co-N/C nanocomposite, which exhibits a porous structure with a high specific area and uniform Co nanoparticle distribution in nitrogen-doped graphite. In addition, the Co-N/C catalysts possess high cobalt content (23%) with highly active β-Co as the main existing form and high nitrogen content (3%). These interesting characteristics endow the Co-N/C nanocomposite with excellent catalytic activity for the hydrogenation reduction of nitro compounds under mild conditions. In addition, the obtained Co-N/C nanocomposites possess a broad substrate scope and good cycle stability for the reduction of halogen-substituted or carbonyl substituted phenyl nitrates. This journal is