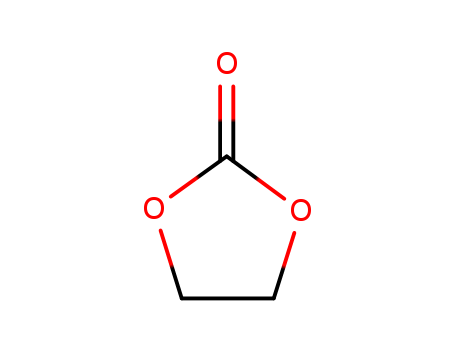
- Chemical Name:Ethylene carbonate
- CAS No.:96-49-1
- Molecular Formula:C3H4O3
- Molecular Weight:88.063
- Hs Code.:29209010
- European Community (EC) Number:202-510-0
- NSC Number:16568,11801
- UNII:RGJ96TB7R7
- DSSTox Substance ID:DTXSID2026600
- Nikkaji Number:J360C
- Wikipedia:Ethylene_carbonate
- Wikidata:Q421145
- ChEMBL ID:CHEMBL3181803
- Mol file:96-49-1.mol
Synonyms:1,3-dioxolan-2-one;ethylene carbonate


 Xi
Xi


