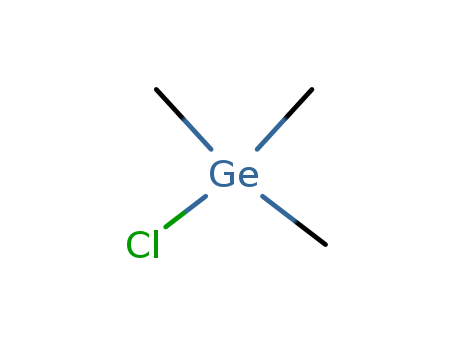
- Chemical Name:Chlorotrimethylgermane
- CAS No.:1529-47-1
- Molecular Formula:C3H9ClGe
- Molecular Weight:153.147
- Hs Code.:2931900090
- European Community (EC) Number:216-214-4
- DSSTox Substance ID:DTXSID70165155
- Nikkaji Number:J47.892J
- Wikidata:Q19756533
- Mol file:1529-47-1.mol
Synonyms:Chlorotrimethylgermane;Trimethylgermanium chloride;1529-47-1;Trimethylchlorogermane;chloro(trimethyl)germane;EINECS 216-214-4;MFCD00000462;C3H9ClGe;chlorotrimethylgermanium;Trimethylgermyl chloride;(CH3)3GeCl;C3-H9-Cl-Ge;Chlorotrimethylgermane, 98%;SCHEMBL302433;Trimethylgermanium(IV)chloride;DTXSID70165155;AKOS015840102;CS-0447183;FT-0722645;T1161;T71831;Q19756533


 F,
F,  C
C


