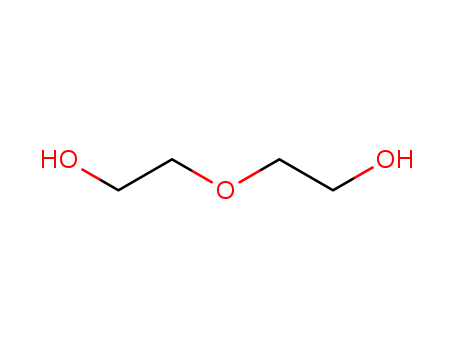
- Chemical Name:Diethylene glycol
- CAS No.:111-46-6
- Deprecated CAS:4669-26-5,2126734-27-6
- Molecular Formula:C4H10O3
- Molecular Weight:106.122
- Hs Code.:2909.41
- European Community (EC) Number:203-872-2
- ICSC Number:0619
- NSC Number:36391,35746,35745,35744,32856,32855
- UNII:61BR964293
- DSSTox Substance ID:DTXSID8020462
- Nikkaji Number:J5.110A
- Wikipedia:Diethylene glycol,Diethylene_glycol
- Wikidata:Q421902
- Metabolomics Workbench ID:52447
- ChEMBL ID:CHEMBL1235226
- Mol file:111-46-6.mol
Synonyms:diethylene glycol


 Xn,
Xn, T,
T, Xi
Xi


