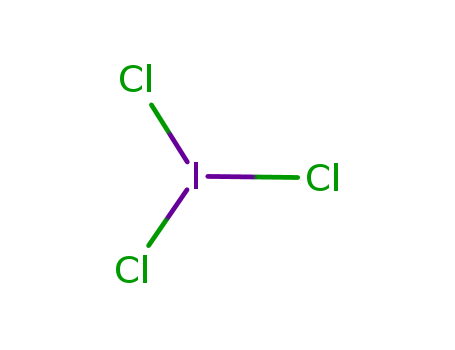
- Chemical Name:Iodine trichloride
- CAS No.:865-44-1
- Molecular Formula:Cl3I
- Molecular Weight:233.264
- Hs Code.:28121099
- European Community (EC) Number:212-739-8
- UNII:1E5KQ66TRQ
- DSSTox Substance ID:DTXSID3061219
- Nikkaji Number:J96.352F
- Wikipedia:Iodine(III) chloride,Iodine trichloride,Iodine_trichloride
- Wikidata:Q419622
- Mol file:865-44-1.mol
Synonyms:Iodine trichloride;865-44-1;Iodine chloride (ICl3);trichloroiodine;trichloro-lambda3-iodane;UNII-1E5KQ66TRQ;1E5KQ66TRQ;EINECS 212-739-8;Cl3I;Jodtrichlorid;Cl3-I;Iodine trichloride, 97%;IODINE TRICHLORIDE [MI];DTXSID3061219;IODINE TRICHLORIDE [INCI];MFCD00036291;AKOS015853996;Iodine trichloride, SAJ first grade, >=96.0%;Iodine trichloride, SAJ special grade, >=97.0%;Q419622


 T,
T, C
C


