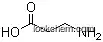Products Categories
| CAS No.: | 110-16-7 |
|---|---|
| Name: | Maleic acid |
| Article Data: | 402 |
| Molecular Structure: | |
|
|
|
| Formula: | C4H4O4 |
| Molecular Weight: | 116.073 |
| Synonyms: | cis-1,2-Ethylenedicarboxylic acid;cis-2-Butenedioicacid;cis-Butenedioic acid;cis-Butene dioic acid;2-Butenedioicacid (Z)-;Maleic acid (8CI);(2Z)-Butene-2-dioic acid;(Z)-2-Butenedioic acid;2-Butenedioic acid, (Z)-;Malezid CM;Scotchbond MultipurposeEtchant;Toxilic acid;(2Z)-but-2-enedioic acid;(2Z)-2-Butenedioic acid; |
| EINECS: | 203-742-5 |
| Density: | 1.499 g/cm3 |
| Melting Point: | 137-140 °C(lit.) |
| Boiling Point: | 355.5 °C at 760 mmHg |
| Flash Point: | 183 °C |
| Solubility: | 790 g/L (25 °C) in water |
| Appearance: | White solid |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 22-36/37/38 |
| Safety: | 26-28-37 |
| Transport Information: | UN 2215 |
| PSA: | 74.60000 |
| LogP: | -0.28820 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID

| Conditions | Yield |
|---|---|
| With formic acid; dihydrogen peroxide at 100℃; for 1h; | 99% |
| With air; vanadia at 320℃; | |
| With air; Bismuth vanadate at 320℃; |

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In water at 20℃; for 6h; Electrolysis; | 95.2% |
| With dihydrogen peroxide; potassium bromide; potassium hydroxide In water at 100℃; for 3h; Reagent/catalyst; | 87.1% |
| With formic acid; dihydrogen peroxide In water at 79.84℃; under 760.051 Torr; for 24h; | 22.4% |

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 20℃; under 760.051 Torr; for 5.5h; Green chemistry; | 94% |
| With hydrogen In methanol under 760.051 Torr; for 6h; | 92% |
| With water; palladium Hydrogenation; |

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In water at 20℃; for 6h; Electrolysis; | 90.7% |
| With formic acid; dihydrogen peroxide at 100℃; for 1h; | 6% |
| With dihydrogen peroxide In water at 80℃; for 5h; | 40 %Chromat. |

| Conditions | Yield |
|---|---|
| With formic acid; dihydrogen peroxide at 100℃; for 0.666667h; Mechanism; Kinetics; Reagent/catalyst; Temperature; Time; Sealed tube; Green chemistry; | 90% |
| With hierarchical cobalt substituted aluminophosphate molecular sieves synthesized using 0.45 % CTAB as template at 60℃; for 3h; Reagent/catalyst; | 86.9% |
| With dihydrogen peroxide; acetic acid; methyltrioxorhenium(VII) In water at 20℃; | 81% |

| Conditions | Yield |
|---|---|
| With tetrafluoroboric acid; dihydrogen peroxide; 5 weight percent methyltrioxorhenium on polystyrene In water at 20℃; for 24h; Product distribution / selectivity; | A 10% B 90% |
| With dihydrogen peroxide In water at 79.84℃; under 760.051 Torr; for 24h; | A 72.1% B 13.8% |
| With dihydrogen peroxide In water at 79.84℃; under 760.051 Torr; for 24h; Reagent/catalyst; Schlenk technique; Green chemistry; | A 74 %Chromat. B 11 %Chromat. |
| With hydrogenchloride In water at 80℃; for 5h; Reagent/catalyst; | A 22 %Chromat. B 34 %Chromat. |
| With zinc(II) nitrate hexahydrate; dihydrogen peroxide In water at 80℃; for 5h; Reagent/catalyst; | A 18 %Chromat. B 13 %Chromat. |

| Conditions | Yield |
|---|---|
| With formic acid; dihydrogen peroxide at 100℃; for 1h; | 89% |
| With potassium hydrogencarbonate In water at 20℃; for 6h; Electrolysis; | 49.3% |
| With sulfuric acid In water at 60℃; pH=1; Electrochemical reaction; | 35.5% |
| With dihydrogen peroxide In water at 80℃; for 5h; | 28 %Chromat. |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; 5 weight percent methyltrioxorhenium on polystyrene In water at 20℃; Product distribution / selectivity; | A 87% B 1% |
| With hydrogenchloride; sodium chlorite; sodium dihydrogenphosphate; dihydrogen peroxide In water; acetonitrile at 10℃; for 1h; | A 82% B 15% |
| With dihydrogen peroxide; 5 weight percent methyltrioxorhenium on polystyrene In water at 20℃; for 24h; Product distribution / selectivity; | A 18% B 82% |
| With water; dihydrogen peroxide at 60℃; for 4h; pH=7.5; | A 45 %Spectr. B n/a |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid; 5 weight percent methyltrioxorhenium on polystyrene In water at 20℃; Product distribution / selectivity; | A 9% B 84% |
- 6118-51-0
exo-3,6-epoxy-1,2,3,6-tetrahydrophthalic anhydride

A
- 108-31-6
maleic anhydride

B
- 110-16-7
maleic acid

| Conditions | Yield |
|---|---|
| With formic acid In water; acetonitrile at 90℃; for 5h; Reagent/catalyst; | A 82% B 7% |
- 59-43-8Thiamine chloride
- 112-02-71-Hexadecanaminium,N,N,N-trimethyl-, chloride (1:1)
- 58-32-2Dypyridamole
- 57808-66-9Domperidone
- 7786-81-4Sulfuric acid,nickel(2+) salt (1:1)
- 104-88-14-Chlorobenzaldehyde
- 151096-09-23-Quinolinecarboxylicacid,1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-
- 12150-46-81,1'-Bis(diphenylphosphino)ferrocene
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Consensus Reports
Standards and Recommendations
Specification
The Maleic acid, with the CAS registry number 110-16-7 and EINECS registry number 203-742-5, has the IUPAC name of (Z)-but-2-enedioic acid. And the molecular formula of this chemical is C4H4O4. It is a kind of white solid, and belongs to the product category of Miscellaneous. This dicarboxylic acid is the cis isomer of butenedioic acid, whereas fumaric acid is the trans isomer.
The physical properties of Maleic acid are as following: (1)ACD/LogP: -0.01; (2)# of Rule of 5 Violations: 0; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 4; (8)#H bond donors: 2; (9)#Freely Rotating Bonds: 2; (10)Polar Surface Area: 52.6 Å2; (11)Index of Refraction: 1.526; (12)Molar Refractivity: 23.76 cm3; (13)Molar Volume: 77.4 cm3; (14)Polarizability: 9.42×10-24cm3; (15)Surface Tension: 67.6 dyne/cm; (16)Density: 1.499 g/cm3; (17)Flash Point: 183 °C; (18)Enthalpy of Vaporization: 65.99 kJ/mol; (19)Boiling Point: 355.5 °C at 760 mmHg; (20)Vapour Pressure: 5.19E-06 mmHg at 25°C.
Preparation of Maleic acid: In industry, it is derived by hydrolysis of maleic anhydride, the latter being produced by oxidation of benzene or butane. It can also be prepared by the hydrolyzation of maleic anhydride, and the maleic anhydride can be obtained from malic acid in the presence of acetyl chloride.
.jpg)
Uses of Maleic acid: It is mainly used as a precursor to fumaric acid, and relative to its parent maleic anhydride, maleic acid has few applications. It is also used in pesticide marathon, fumaric acid, unsaturated polyester and dyeing auxiliary.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin, and it is also harmful if swallowed. Therefore, you had better take the following instructions: Wear suitable gloves, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)\C=C/C(=O)O
(2)InChI: InChI=1/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1-
(3)InChIKey: VZCYOOQTPOCHFL-UPHRSURJBG
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 2400mg/kg (2400mg/kg) | Biochemical Journal. Vol. 34, Pg. 1196, 1940. | |
| rabbit | LD50 | skin | 1560mg/kg (1560mg/kg) | BEHAVIORAL: TREMOR | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 7-4/1970, |
| rat | LC50 | inhalation | > 720mg/m3/1H (720mg/m3) | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 7-4/1970, | |
| rat | LD50 | oral | 708mg/kg (708mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: MUSCLE WEAKNESS GASTROINTESTINAL: ULCERATION OR BLEEDING FROM STOMACH | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 7-4/1970, |