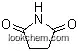
Products Categories
| CAS No.: | 96-48-0 |
|---|---|
| Name: | gamma-Butyrolactone |
| Article Data: | 494 |
| Molecular Structure: | |
|
|
|
| Formula: | C4H6O2 |
| Molecular Weight: | 86.0904 |
| Synonyms: | 1,4-Butanolide;1-Oxacyclopentan-2-one;2,3,4,5-Tetrahydro-2-furanone;2-Oxolanone;2-Oxotetrahydrofuran;4,5-Dihydro-2(3H)-furanone;4-Butanolide;4-Deoxytetronicacid;4-Hydroxybutanoic acid lactone;4-Hydroxybutyric acid lactone;Butanoicacid, 4-hydroxy-, g-lactone;Butyric acid lactone;Butyrolactone;Dihydro-2(3H)-furanone;NIH 10540;NSC 4592;Paint Clean G;Tetrahydro-2-furanone;g-BL;g-Butalactone;g-Butyrolactone;g-Butyryllactone;g-Hydroxybutyric acid lactone;1,4-Butyrolactone;gamma-Butyrolactone; |
| EINECS: | 202-509-5 |
| Density: | 1.128 g/cm3 |
| Melting Point: | -45 °C(lit.) |
| Boiling Point: | 204 °C at 760 mmHg |
| Flash Point: | 80.9 °C |
| Solubility: | MISCIBLE |
| Appearance: | colourless oily liquid |
| Hazard Symbols: |
 Xn Xn
|
| Risk Codes: | 22-36 |
| Safety: | 26-36-39 |
| PSA: | 26.30000 |
| LogP: | 0.32340 |
- 99170-93-1N-Methyl-2-oxazolamine
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile
- 98020-47-46-Amino-5-nitro-2-thio-uracil
- 97271-97-12-AMINO-3-NITRO-BENZALDEHYDE
- 97484-76-91-(prop-2-ynyl)-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione
- 97484-75-81-allyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione
- 98025-62-8(5-METHYL-[1,3,4]THIADIAZOL-2-YL)-HYDRAZINE
- 99724-17-1Dimethyl-pyrrolidin-3-ylmethyl-amine
- 97484-82-71-allyl-7-methyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione
- 96949-46-12,4-dichloro-5-iodo-6-methylpyrimidine

| Conditions | Yield |
|---|---|
| With 5 wt% Pd nanoparticles loaded on phosphate anion exchanged [Mg6Al2(OH)16]CO3*xH2O; air at 50℃; under 760.051 Torr; for 6h; Reagent/catalyst; Irradiation; | 100% |
| With acetone; dihydridotetrakis(triphenylphosphine)ruthenium In toluene at 180℃; for 3h; | 88 % Chromat. |

| Conditions | Yield |
|---|---|
| With 5% active carbon-supported ruthenium; isopropyl alcohol at 160℃; for 0.416667h; Reagent/catalyst; Temperature; Microwave irradiation; | 100% |
| With hydrogen In 1,4-dioxane at 180℃; under 37503.8 Torr; for 3h; Reagent/catalyst; Solvent; Autoclave; |

| Conditions | Yield |
|---|---|
| With hydrogen; Cu-based catalyst at 210℃; Product distribution; Further Variations:; Temperatures; reaction in vapour phase, fixed bed reactor, coupled dehydrogenation reactions of title comp. and INO 160; | A 96.5% B 99.4% |


| Conditions | Yield |
|---|---|
| With oxone; silica gel In dichloromethane at 20℃; for 1h; Baeyer-Villiger oxidation; | 99% |
| With acyltransferase from Mycobacterium smegmatis; dihydrogen peroxide; ethyl acetate In water at 35℃; for 2h; Baeyer-Villiger Ketone Oxidation; Enzymatic reaction; | 99% |
| With 2,2,2-trifluoroethanol; dihydrogen peroxide for 24h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With C16H25N3O2S In n-heptane for 3h; Reflux; Molecular sieve; Inert atmosphere; | 99% |
| With C16H25N3O2S In n-heptane for 48h; Reflux; Molecular sieve; | 99% |
| In hexane at 26℃; porcine pancreatic lipase (PPL); | |
| With porcine pancreatic lipase (E.(1)C313) In diethyl ether at 26℃; Yield given; |

| Conditions | Yield |
|---|---|
| With hydrogen In methanol under 26252.6 Torr; for 2h; Reagent/catalyst; Autoclave; | 99% |
| With 0.5% palladium on silica gel; hydrogen In methanol at 80℃; under 26252.6 Torr; Catalytic behavior; Kinetics; Reagent/catalyst; Autoclave; | 92.6% |
| With Ni#NiO; hydrogen In ethanol at 80℃; under 22502.3 Torr; for 1h; Reagent/catalyst; Autoclave; | 86.6% |
- 110-16-7
maleic acid

A
- 109-99-9
tetrahydrofuran

B
- 96-48-0
4-butanolide

C
- 110-63-4
Butane-1,4-diol

D
- 110-15-6
succinic acid

E
- 64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5 percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.37% B 0.28% C 0.37% D 98.89% E 0.08% |


| Conditions | Yield |
|---|---|
| With hydrogen; Cu-based catalyst at 190℃; Product distribution; Further Variations:; Temperatures; reaction in vapour phase, fixed bed reactor; | A 98.8% B 0.8% |
- 110-16-7
maleic acid

A
- 109-99-9
tetrahydrofuran

B
- 96-48-0
4-butanolide

C
- 67-56-1
methanol

D
- 110-63-4
Butane-1,4-diol

E
- 617-48-1
malic acid

F
- 110-15-6
succinic acid

G
- 64-19-7
acetic acid

H
- 71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.45% B 0.06% C 0% D 0.21% E 0.36% F 98.73% G 0.04% H 0.08% |

- 110-16-7
maleic acid

A
- 109-99-9
tetrahydrofuran

B
- 96-48-0
4-butanolide

C
- 110-63-4
Butane-1,4-diol

D
- 591-81-1
4-hydroxybutanoic acid

E
- 110-15-6
succinic acid

F
- 64-19-7
acetic acid

G
- 71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; 0.5percent Pd on Rutile TiO2 at 110℃; Product distribution / selectivity; | A 0.77% B 0.38% C 0.24% D 0.05% E 98.28% F 0.02% G 0.26% |

 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 56.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal No Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 11 (1976),p. 231.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . EPA Genetic Toxicology Program. Reported in EPA TSCA Inventory.
Specification
The gamma-Butyrolactone is an organic compound with the formula C4H6O2. The IUPAC name of this chemical is oxolan-2-one. With the CAS registry number 96-48-0, it is also named as 1-Oxacyclopentan-2-one. The product's category is Pharmaceutical Intermediates. Besides, gamma-Butyrolactone (γ-butyrolactone or GBL) is a hygroscopic colorless oily liquid with a weak characteristic odor and is soluble in water, which should be stored in a cool and ventilated place.
Physical properties about gamma-Butyrolactone are: (1)ACD/LogP: -0.76; (2)ACD/LogD (pH 5.5): -0.76; (3)ACD/LogD (pH 7.4): -0.76; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 9.15; (7)ACD/KOC (pH 7.4): 9.15; (8)#H bond acceptors: 2; (9)Polar Surface Area: 26.3 Å2; (10)Index of Refraction: 1.442; (11)Molar Refractivity: 20.18 cm3; (12)Molar Volume: 76.2 cm3; (13)Polarizability: 8×10-24cm3; (14)Surface Tension: 35.4 dyne/cm; (15)Density: 1.128 g/cm3; (16)Flash Point: 80.9 °C; (17)Enthalpy of Vaporization: 44.02 kJ/mol; (18)Boiling Point: 204 °C at 760 mmHg; (19)Vapour Pressure: 0.27 mmHg at 25°C.
Preparation of gamma-Butyrolactone: gamma-Butyrolactone can be prepared by gamma-hydroxybutyric acid (GHB) by removal of water or by distillation from such a mixture. It may also be obtained via oxidation of tetrahydrofuran (THF). One such process, which affords GBL in yields of up to 80%, utilises bromine generated in situ from an aqueous solution of sodium bromate and potassium hydrogen sulfate. Another process can proceed by using commercially-available calcium hypochlorite in the presence of activating acetic acid and an appropriate solvent such as acetonitrile.
Uses of gamma-Butyrolactone: GHB (gamma hydroxybutyrate) and GBL (gamma butyrolactone) are substances which are often used as recreational drugs. GHB has two effects, at low doses it has a euphoric effect (which is why it is sometimes referred to as liquid ecstasy). At higher doses it acts like a sedative and can make the user unconscious very quickly.
When you are using this chemical, please be cautious about it as the following: It is harmful if swallowed. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Besides, this chemical is irritating to eyes. When you are using it, wear suitable protective clothing and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C1OCCC1
(2)InChI: InChI=1/C4H6O2/c5-4-2-1-3-6-4/h1-3H2
(3)InChIKey: YEJRWHAVMIAJKC-UHFFFAOYAC
(4)Std. InChI: InChI=1S/C4H6O2/c5-4-2-1-3-6-4/h1-3H2
(5)Std. InChIKey: YEJRWHAVMIAJKC-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | skin | > 5gm/kg (5000mg/kg) | GAF Material Safety Data Sheet. | |
| mouse | LD50 | intraperitoneal | 1100mg/kg (1100mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Archivum Immunologiae et Therapiae Experimentalis. Vol. 13, Pg. 70, 1965. |
| mouse | LD50 | oral | 1460mg/kg (1460mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 64(2), Pg. 3, 1999. |
| mouse | LDLo | parenteral | 1600mg/kg (1600mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 7, Pg. 687, 1955. | |
| rabbit | LDLo | intravenous | 500mg/kg (500mg/kg) | Archivum Immunologiae et Therapiae Experimentalis. Vol. 13, Pg. 70, 1965. | |
| rat | LC50 | inhalation | > 5100mg/m3/4H (5100mg/m3) | National Technical Information Service. Vol. OTS0534527, | |
| rat | LD50 | intraperitoneal | 1gm/kg (1000mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Archivum Immunologiae et Therapiae Experimentalis. Vol. 13, Pg. 70, 1965. |
| rat | LD50 | oral | 1540mg/kg (1540mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(1), Pg. 49, 1987. |