Products Categories
| CAS No.: | 114798-26-4 |
|---|---|
| Name: | Losartan |
| Article Data: | 55 |
| Molecular Structure: | |
|
|
|
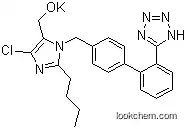
| Formula: | C22H23ClN6O |
| Molecular Weight: | 422.917 |
| Synonyms: | 2-n-butyl-4-chloro-5-hydroxymethyl-1-[[2-(1H-tetrazol-5-yl)-biphenyl-4-yl]methyl]imidazole;DUP 89;1H-imidazole-5-methanol, 2-butyl-4-chloro-1-[[2-(2H-tetrazol-5-yl)[1,1-biphenyl]-4-yl]methyl]-;[2-butyl-5-chloro-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol;2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5ylphenyl)benzyl]imidazole-5-methanol;2-Butyl-4-chloro-1-[[2-(1H-tetrazol-5-yl)[1,1-biphenyl]-4-yl]methyl-1H-imidazole-5-methanol;Losartan Base; |
| EINECS: | 601-329-8 |
| Density: | 1.35 g/cm3 |
| Melting Point: | 183-184 °C |
| Boiling Point: | 682 °C at 760mmHg |
| Flash Point: | 366.3 °C |
| Solubility: | 4.8mg/L at 20℃ |
| Appearance: | pale yellow solid |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 36/37/38 |
| Safety: | 26-36 |
| PSA: | 92.51000 |
| LogP: | 4.26680 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID
- 114772-55-3
2-n-Butyl-4-chloro-1-[(2'-cyanobiphenyl-4-yl)-methyl]-5-(hydroxymethyl)imidazole

- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| With sodium azide In 1-methyl-pyrrolidin-2-one | 99% |
| With sodium azide In toluene at 20℃; | 95% |
| With sodium azide; zinc trifluoromethanesulfonate In water at 100℃; for 6h; Solvent; Temperature; Time; Green chemistry; | 91% |
- 67-56-1
methanol

- 124751-00-4
2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol

A
- 596-31-6
methoxytriphenylmethane

B
- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| for 7h; Heating / reflux; | A 90% B 98% |


- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen at 20℃; under 760.051 Torr; for 24h; | 97% |
| With palladium on carbon; hydrogen; acetic acid at 20℃; for 12h; | 94% |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / methanol; toluene / 0.5 h / 25 °C / Inert atmosphere 2: ammonium formate; 5% palladium on barium sulphate / isopropyl alcohol; water / 8 h / 25 - 60 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate; methanol / toluene / 0.58 h / 0 - 25 °C 2: 5% palladium on barium sulphate; ammonium formate / water; isopropyl alcohol / 8 h / 60 °C View Scheme |
- 124751-00-4
2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol

A
- 596-31-6
methoxytriphenylmethane

B
- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| Stage #1: 2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol With hydroxylamine hydrochloride In methanol; water; acetone at 20℃; for 2h; Stage #2: With sodium hydroxide In methanol; water; acetone at 20 - 25℃; pH=3.8 - 4.2; | A n/a B 95% |
| Stage #1: 2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol With hydrogenchloride In methanol; water; acetone at 20℃; for 2h; Stage #2: With sodium hydroxide In methanol; water; acetone at 20 - 25℃; pH=3.8 - 4.2; | A n/a B 93% |
| Stage #1: 2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol With sulfuric acid In methanol; water; acetone at 20℃; for 2h; Stage #2: With sodium hydroxide In methanol; water; acetone at 20 - 25℃; pH=3.8 - 4.2; | A n/a B 91.3% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol With hydroxylamine hydrochloride In methanol; water; acetone at 20℃; for 2h; Stage #2: With sodium hydroxide In methanol; water; acetone at 20 - 25℃; pH=3.8 - 4.2; Stage #3: ethyl acetate at 40℃; for 1h; | A n/a B n/a C 95% |

- 124751-00-4
2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol

- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| Stage #1: 2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol; hydroxylamine hydrochloride In methanol at 60℃; for 2.5h; pH=2.95; Stage #2: With triethylamine In methanol at 0 - 40℃; for 1h; pH=3.6; Product distribution / selectivity; | 94.7% |
| Stage #1: 2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol; hydroxyammonium sulfate In isopropyl alcohol at 60 - 65℃; for 4h; pH=2.4; Stage #2: With triethylamine In isopropyl alcohol at 0 - 5℃; for 2h; pH=3.5; Product distribution / selectivity; | 93.8% |
| With hydrogenchloride; water In tetrahydrofuran at 20℃; for 4h; | 90% |

- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| With sulfuric acid In water; ethyl acetate at 10 - 25℃; for 1h; pH=3.6 - 3.8; Purification / work up; | 94% |
- 124750-99-8
cozaar

- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| With sulfuric acid In water; ethyl acetate at 10 - 25℃; for 1h; pH=3.6 - 3.8; Purification / work up; | 93% |
| With hydrogenchloride In tetrahydrofuran; water; chlorobenzene | 86.8% |
| With hydrogenchloride In tetrahydrofuran; water; chlorobenzene pH=3.5 - 3.6; | 84% |
| With hydrogenchloride In tetrahydrofuran; water; chlorobenzene pH=3.5 - 3.6; | 42.6 g |

- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water; acetonitrile at 15 - 25℃; pH=3.5 - 3.6; | 93% |
- 133909-99-6
2-n-butyl-4-chloro-1-[(2'-(2-triphenylmethyl-2H-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl]-1H-imidazole-5-methanol

- 114798-26-4
lorsartan

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran at 25℃; for 4h; | 89% |
- 269055-15-4Etravirine
- 108-45-2m-Phenylenediamine
- 286930-03-8Fesoterodine fumarate
- 3282-30-2Pivaloyl chloride
- 532-43-4Thiamine nitrate
- 10279-57-9Silica hydrate
- 122-39-4Diphenylamine
- 100643-71-8Desloratadine
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Specification
Losartan, with its IUPAC Name of [2-Butyl-5-chloro-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol, is one kind of pale yellow solid. And it belongs to the Classification Code which include Angiotensin II Type 1 Receptor Blockers; Anti-arrhythmia agents; Antihypertensive agents; Cardiovascular Agents; Drug / Therapeutic Agent; Human Data. Losartan is used mainly to treat high blood pressure (hypertension). Losartan is being researched as a possible drug for marked slowing of aortic enlargement in Marfan and related syndromes.
Physical properties about Losartan are: (1)ACD/LogP: 4.364; (2)ACD/LogD (pH 5.5): 3.13; (3)ACD/LogD (pH 7.4): 2.39; (4)ACD/BCF (pH 5.5): 71.72; (5)ACD/BCF (pH 7.4): 13.05; (6)ACD/KOC (pH 5.5): 331.15; (7)ACD/KOC (pH 7.4): 60.25; (8)#H bond acceptors: 7; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 9; (11)Index of Refraction: 1.681; (12)Molar Refractivity: 118.236 cm3; (13)Molar Volume: 312.526 cm3; (14)Polarizability: 46.872 10-24cm3; (15)Surface Tension: 53.3219985961914 dyne/cm; (16)Density: 1.353 g/cm3; (17)Flash Point: 366.27 °C; (18)Enthalpy of Vaporization: 105.096 kJ/mol; (19)Boiling Point: 682.016 °C at 760 mmHg
Production of Losartan: Losartan could be produced through the following simple way:
.gif)
You can still convert the following datas into molecular structure:
1). InChI: InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28)
2). InChIKey: InChIKey=PSIFNNKUMBGKDQ-UHFFFAOYSA-N
3). Smiles: n1(c(nc(c1CO)Cl)CCCC)Cc1ccc(c2c(c3nnn[nH]3)cccc2)cc1