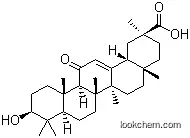
471-53-4 Usage
Description
Different sources of media describe the Description of 471-53-4 differently. You can refer to the following data:
1. Extracting from liquorice (Glycyrrhiza uralensis Fisch, Gan Cao), glycyrrhetic acid
also can be detected in other plants, such as Abrus cantoniensis Hance and Herba
Abri fruticulosi. As one of traditional Chinese medicines, liquorice has been applied
clinically for a long period. Due to its extensive usage, it plays an extremely important role in traditional Chinese medicine formula mainly as “guide” drug. As is
recorded in Shen Nong’s Herbal Classic and later in pharmaceutical monographs,
liquorice is able to strengthen bones and muscles and enhance metabolism and
detoxification. Also, abnormal symptoms of the body and wound can be improved.
Glycyrrhetic acid, the most important and potent ingredient of liquorice, has been
recorded in Pharmacopoeia of the People’s Republic of China.
2. 18β-Glycyrrhetinic acid is a major metabolite of glycyrrhizin , one of the main constituents of licorice. Both 18β-glycyrrhetinic acid and glycyrrhizin have been shown to exhibit anti-ulcerative, anti-inflammatory, and immunomodulatory properties. 18β-Glycyrrhetinic acid is an inhibitor of the complement pathway (IC50 = 35 μM). At 100 mg/kg/day, 18β-glycyrrhetinic acid is protective against diabetes complications by reducing lipid peroxidation and increasing antioxidant activity in diabetic rats. 18β-Glycyrrhetinic acid inhibits mammalian DNA polymerases α, γ, κ, and λ with IC50 values of 16.1, 19.3, 15.8, and 13.7 μM, respectively. At 100-200 μM, 18β-glycyrrhetinic acid suppresses LPS-induced TNF-α production and NF-κB activation in mouse macrophages.
Chemical Properties
white or greyish-white crystalline powder
Physical properties
Solubility: insoluble in water; it exists in crystal with methanol and chloroform.
Melting point: the compound melts at 292–295?°C. Specific optical rotation: under
the condition of 20?°C, 589.3?nm, and 1?dm, polarized light rotates at 68° when it
passes through the chloroform with a concentration of 64? mol/L.? Both
18α-glycyrrhetic acid and 18β-glycyrrhetic acid are chiral isomers of glycyrrhetic
acid.
History
Glycyrrhetic acid originates from hydrolysis of glycyrrhizin, which has a therapeutic effect on disease. Dating back to the 1930s, the chemical structure of glycyrrhetic acid was demonstrated . Subsequently, the discovery of antiulcer activity
promotes following research . The ramification of glycyrrhetic acid, carbenoxolone sodium, has a therapeutic effect on ulcer. In 2010, followed by the approval of
raw materials, batches of tablets and capsules were approved in 2009, respectively.
In foreign countries, 18β-glycyrrhetic acid was studied for anti-inflammatory effect
on arthritis, rheumatoid disease, and periodontitis in BioNetWorks. The company
applied for the patent of 18β-glycyrrhetic acid in 1999. Also, after joining the leading worldwide market in 2006, phase III clinical trials would be carried out in 2007.
However, the progress was hindered in 2008.
To detecting more indications, its carbenoxolone sodium was studied by other
three companies: after conducting phase III clinical trials in the UK, the project of
RB intending to improve nonspecific inflammatory bowel disease was given up in
1992. York Pharma expected to make progress in psoriasis with gel or cream; however, the project has been in a standstill after phase II clinical trials was conducted
from 2005 to 2009. Canada pharmaceutical company, Oxalys Pharmaceuticals,
research it for treating Huntington’s disease, and it was included in the orphan drug
list by the USA in 2014. Till now, phase I clinical trials are still continuing.
Uses
Different sources of media describe the Uses of 471-53-4 differently. You can refer to the following data:
1. The product may be used as a starting material to prepare 18β-glycyrrhetinic acid derivatives, which show anti-inflammatory and antioxidant properties.
2. An anti-inflammatory (topical).
3. antitussive, antiinflammatory, antibacterial
4. The aglycone of the triterpenoid Glyccyrrhizic acid.
5. glycyrrhetinic acid is anti-irritant, anti-allergenic, anti-inflammatory, skin-lightening, and smoothing properties are attributed to this ingredient, which is also a carrier. It is the organic compound derived from glycyrrhizic acid or shredded licorice roots.
Definition
ChEBI: A pentacyclic triterpenoid that is olean-12-ene substituted by a hydroxy group at position 3, an oxo group at position 11 and a carboxy group at position 30.
Indications
Treatment of Addison’s disease, deoxycorticosterone
General Description
18β-Glycyrrhetinic acid is a pentacyclic triterpenoid found in the Glycyrrhiza glabra L.(liquorice) roots. It is the key metabolite of glycyrrhizin and glycyrrhizic acid.
Pharmacology
Thirty percent of glycyrrhetinic acid can be effectively used by the body; both 18α-glycyrrhetic acid and 18β-glycyrrhetic acid reduce by half in 2.24 h and 11.5 h separately. CYP3A promotes metabolism with hydroxyl added to 22α and 24α .There are lots of pharmacological activities : it plays an anti-inflammatory role by inhibiting the activity of phospholipase A2 and lipoxygenase to reduce mediators of inflammation; the compound promotes antiulcer activity through the production of more PGE2 and secretion of gastric mucus; it also provokes proliferation of gastric cell to protect the mucosa from ulceration. The complex which consists of glycyrrhetinic acid and carotenoid plays antioxidation by scavenging free radical. Glycyrrhetinic acid inhibits the replication of viral DNA to achieve an antiviral effect at the concentration of 4×10?5 mol/L; it also inhibits proliferation of tumor cell and promotes apoptosis and differentiation. The decreasing ability of invasion exerts an antitumor effect. Glycyrrhetinic acid is considered to have extensive antiarrhythmic effects through inhibition of L-type calcium channel. In addition, glycyrrhetinic acid functions as an anticholinesterase (1.7×10?5 mol/L), anticoagulant, and antitetanus toxin; it also improves inner ear hearing (100 mg/kg, intramuscular injection) and improves absorption of insulin.
Anticancer Research
Different sources of media describe the Anticancer Research of 471-53-4 differently. You can refer to the following data:
1. Glycyrrhetinic acid in combinationwith etoposide inhibits thetopoisomerase 2α and inducesapoptosisCai et al.(2017)
2. It was reported that GA at noncytotoxic concentrationshowed synergistic effect in combination with anticancer drug, etoposide (VP-16).Specifically, GA enhanced cytotoxicity through regulating topoisomerase II-αtargeted by etoposide. Also, GA sensitized the cells to etoposide through elevatingtopoisomerase II-α with a 2.4-fold rate at 12 h time point. From 12 to 48 h, GAhalved the expression of topoisomerase II-α and stimulated apoptosis, whichexhibited its antineoplastic effect. They reported that GA was more potentiallyeliminating the TNBC cells when compared with Glycyrrhizin Acid (Cai et al. 2017).
Clinical Use
Glycyrrhetic acid has not been applied in clinical treatment till now. Meanwhile, the
ramification has come into the market for the property of antiulcer. However, with
large doses and long-term usage, the drug gives rise to hypertension, sodium retention, and hypokalemia. When renin-angiotensin-aldosterone system fails to function properly, liquorice-induced pseudoaldosteronism threatens human health .
Check Digit Verification of cas no
The CAS Registry Mumber 471-53-4 includes 6 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 3 digits, 4,7 and 1 respectively; the second part has 2 digits, 5 and 3 respectively.
Calculate Digit Verification of CAS Registry Number 471-53:
(5*4)+(4*7)+(3*1)+(2*5)+(1*3)=64
64 % 10 = 4
So 471-53-4 is a valid CAS Registry Number.
InChI:InChI=1/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21?,22-,23?,26+,27-,28-,29+,30+/m0/s1
471-53-4Relevant articles and documents
Glycyrrhiza glabra extract and quercetin reverses cisplatin resistance in triple-negative MDA-MB-468 breast cancer cells via inhibition of cytochrome P450 1B1 enzyme
Sharma, Rajni,Gatchie, Linda,Williams, Ibidapo S.,Jain, Shreyans K.,Vishwakarma, Ram A.,Chaudhuri, Bhabatosh,Bharate, Sandip B.
, p. 5400 - 5403 (2017)
The development of multi-drug resistance to existing anticancer drugs is one of the major challenges in cancer treatment. The over-expression of cytochrome P450 1B1 enzyme has been reported to cause resistance to cisplatin. With an objective to discover cisplatin-resistance reversal agents, herein, we report the evaluation of Glycyrrhiza glabra (licorice) extracts and its twelve chemical constituents for inhibition of CYP1B1 (and CYP1A1) enzyme in Sacchrosomes and live human cells. The hydroalcoholic extract showed potent inhibition of CYP1B1 in both Sacchrosomes as well as in live cells with IC50 values of 21 and 16 μg/mL, respectively. Amongst the total of 12 constituents tested, quercetin and glabrol showed inhibition of CYP1B1 in live cell assay with IC50 values of 2.2 and 15 μM, respectively. Both these natural products were found to be selective inhibitors of CYP1B1, and does not inhibit CYP2 and CYP3 family of enzymes (IC50 > 20 μM). The hydroalcoholic extract of G. glabra and quercetin (4) showed complete reversal of cisplatin resistance in CYP1B1 overexpressing triple negative MDA-MB-468 breast cancer cells. The selective inhibition of CYP1B1 by quercetin and glabrol over CYP2 and CYP3 family of enzymes was studied by molecular modeling studies.
Oleanane-type triterpene glucuronides from the roots of Glycyrrhiza uralensis Fischer
Zheng, Yun-Feng,Qi, Lian-Wen,Cui, Xiao-Bing,Peng, Guo-Ping,Peng, Yong-Bo,Ren, Mei-Ting,Cheng, Xiao-Lan,Li, Ping
, p. 1457 - 1463 (2010)
Investigation of characteristic constituents of the roots of Glycyrrhiza uralensis Fischer led to isolation of four new triterpene glucuronides, namely uralsaponins CF (1-4), an artificial product, namely the methyl ester of glycyrrhizin (5), as well as six known triterpene glucuronides (6-11). These new compounds were identified by 1D and 2DNMR spectroscopic analysis. The cytotoxicity of the selected compounds and their aglycones were evaluated against HeLa and MCF-7 cancer cell lines, and the preliminary structure-activity relationship was also elucidated.
Bergmann,E.,Bergmann,F.
, p. 208 (1937)
Oestrogenic activity of enoxolone in rodents
Dekanski,Gottfried,MacDonald
, p. 62 - 62 (1979)
-
Application of bacterial directed enzyme prodrug therapy as a targeted chemotherapy approach in a mouse model of breast cancer
Bahrami, Ahmad Reza,Hosseini-Giv, Niloufar,Matin, Maryam M.
, (2021/08/03)
Cancer is the second leading cause of death in the world. Some of the usual cancer treatments include surgery, chemotherapy, and radiotherapy. However, due to low efficacy and side effects of these treatments, novel targeted therapeutic methods are needed. One of the common drawbacks of cancer chemotherapy is off-target toxicity. In order to overcome this problem, many investigations have been conducted. One of the new targeted therapy methods known as bacterial directed enzyme-prodrug therapy (BDEPT) employs bacteria as enzyme carriers to convert a pro-drug to a drug specifically within the tumor site. In the present study, we used Escherichia coli DH5α carrying luxCDABE gene cluster and overexpressing β-glucuronidase for luminescent emission and enzyme expression, respectively. Enzyme expression can lead to the conversion of glycyrrhizic acid as a prodrug to glycyrrhetinic acid, a potent anti-cancer agent. DH5α-lux/βG was characterized and its stability was also evaluated. Bacteria colonization in the tumor site was measured by tissue homogenate preparation and colony counting method. Histopathological studies on the liver, spleen, and tumor were also conducted. According to the results, co-treatment of 4T1, a highly metastatic mouse breast cancer cell line, with GL and DH5α-lux/βG could significantly decrease the IC50 values. Moreover, increased number of bacteria could lead to a dramatic drop in IC50 value. Specific colonization of DH5α-lux/βG was observed in the tumor site compared with other tissues (p 0.0001). Moreover, the biocompatibility evaluation proved that DH5α-lux/βG had no adverse effects on normal tissues. Furthermore, concurrent usage of GL and bacteria in the treatment of induced 4T1 tumors in BALB/c mice significantly delayed tumor growth (p0.001) during 16 days of investigation. Based on these findings, BDEPT might be useful for targeted breast cancer therapy, although further investigations are required to confirm this.
Direct Carbon Isotope Exchange through Decarboxylative Carboxylation
Kingston, Cian,Wallace, Michael A.,Allentoff, Alban J.,Degruyter, Justine N.,Chen, Jason S.,Gong, Sharon X.,Bonacorsi, Samuel,Baran, Phil S.
supporting information, p. 774 - 779 (2019/01/14)
A two-step degradation-reconstruction approach to the carbon-14 radiolabeling of alkyl carboxylic acids is presented. Simple activation via redox-active ester formation was followed by nickel-mediated decarboxylative carboxylation to afford a range of complex compounds with ample isotopic incorporations for drug metabolism and pharmacokinetic studies. The practicality and operational simplicity of the protocol were demonstrated by its use in an industrial carbon-14 radiolabeling setting.
An efficient, scalable approach to hydrolyze flavonoid glucuronides via activation of glycoside bond
Jiang, Xue-Yang,Li, Xin-Chen,Liu, Wen-Yuan,Xu, Yun-Hui,Feng, Feng,Qu, Wei
, p. 1895 - 1903 (2017/03/11)
Hydrolyzing flavonoid glucuronides into corresponding aglycones posed some significant challenges. To improve acid-catalyzed hydrolysis process of flavonoid glucuronide, structures of glucuronide, hydrolysis parameters and post-processing were optimized. The optimized condition was performed by hydrolysis flavonoid glycoside methyl ester in a mixed solvent consisting of 2?mol/L H2SO4/EtOH/H2O (1/8/1, v/v/v) at 95?°C for 7?h and resulted in up to 90% aglycone yields, minimal byproduct formations and milder hydrolysis conditions. Furthermore, the optimized method avoids tedious purification steps and is easily conducted on a relatively large-scale using economical and commercially available reagents.