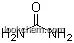Products Categories
| CAS No.: | 1313-99-1 |
|---|---|
| Name: | Nickelous oxide |
| Article Data: | 1813 |
| Molecular Structure: | |
|
|
|
| Formula: | NiO |
| Molecular Weight: | 74.6894 |
| Synonyms: | Mononickel oxide;NiO-D;NiO-G 39;Nickel Oxide Sinter 75;Nickel monooxide;Nickel(II) oxide;Nickelous oxide;Nickel(Ⅱ)oxide,green; |
| EINECS: | 215-215-7 |
| Density: | 6.67 g/mL at 25 °C(lit.) |
| Melting Point: | 1960 °C |
| Solubility: | insoluble in water |
| Appearance: | olive green crystals |
| Hazard Symbols: |
 T, T, Xi Xi
|
| Risk Codes: | 49-43-53 |
| Safety: | 53-45-61-36/37 |
| PSA: | 17.07000 |
| LogP: | -0.11880 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE

| Conditions | Yield |
|---|---|
| In melt Kinetics; byproducts: KCl, NO2, O2; mixt. of K salt/Ni salt (8/1) heated up to 500°C with a rate of 150°C/h, cooled in furnace; washed with H2O, insol. residue filtered off; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In melt Kinetics; byproducts: LiCl, NO2, O2; mixt. of Li salt/Ni salt (8/1) heated up to 500°C with a rate of 150°C/h, cooled in furnace; washed with H2O, insol. residue filtered off; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In melt Kinetics; byproducts: NaCl, NO2, O2; mixt. of Na salt/Ni salt (8/1) heated up to 500°C with a rate of 150°C/h, cooled in furnace; washed with H2O, insol. residue filtered off; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In melt Kinetics; byproducts: KNO3, NO2, O2; mixt. of K salt/Ni salt (8/1) heated up to 500°C with a rate of 150°C/h, cooled in furnace; washed with H2O, insol. residue filtered off; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In melt Kinetics; byproducts: LiNO3, NO2, O2; mixt. of Li salt/Ni salt (8/1) heated up to 500°C with a rate of 150°C/h, cooled in furnace; washed with H2O, insol. residue filtered off; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In melt Kinetics; byproducts: NaNO3, NO2, O2; mixt. of Na salt/Ni salt (8/1) heated up to 500°C with a rate of 150°C/h, cooled in furnace; washed with H2O, insol. residue filtered off; elem. anal.; | 100% |

- 18182-48-4
1-(2-mercaptoethyl)-3,5-dimethylpyrazole

A
- 1313-99-1
nickel(II) oxide

B
- 654670-50-5
[NiCl3(NC(CH3)CHC(CH3)NCH2CH2SSCH2CH2NC(CH3)CHC(CH3)NH)]

| Conditions | Yield |
|---|---|
| With triethyl orthoformate; O2 In acetonitrile vac. line and Schlenk technique; soln. of Ni compd. in CH3CN was added dropwise to soln. of ligand and triethyl orthoformate in CH3CN; soln. waskept in presence of O2 for 3 d; filtered; solvent evapd.; elem. anal.; | A n/a B 96% |


| Conditions | Yield |
|---|---|
| With Dimethylglyoxime; nitric acid In ethanol; ammonia; water byproducts: NH4F; addn. of aq. NH4OH to Ni(PF3)4, cooling with liquid nitrogen, mixing for 15 min, addn. of HNO3, adjusting pH to 7-8 by addn. of NH4OH, heating to 80°C, pptg. by addn. of alcoholicammoniacal soln. of dimethylglyoxime; filtn., heating at 850°C; | 92% |

| Conditions | Yield |
|---|---|
| In solid byproducts: LiCl; under N2; Li2O and metal halide ground together, added to glass ampoule, sealed under vacuum, sonicated (ultrasound) for 10 min, wrapped in glss wool, placed in oven at 450°C for 10 h; removed from oven, allowed to cool to room temp., trituration with THF for 10 h, ppt. and cloudy THF layer formed, evapn.of THF filtrate produced Li salt; or metal oxides washed with H2O for 10 min to remove Li salt; | 75% |
| In neat (no solvent, solid phase) byproducts: LiCl; |

| Conditions | Yield |
|---|---|
| Stage #1: nickel(II) sulfate hexahydrate With ethanol; sodium hydroxide In water Stage #2: With sodium carbonate In water at 80℃; for 24h; Stage #3: air at 450℃; for 2h; Calcination; | 46.9% |
- 74578-69-15-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid, 7-[[(2Z)-2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]-8-oxo-3-[[(1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-1,2,4-triazin-3-yl)thio]methyl]-,sodium salt (1:2), (6R,7R)-
- 152628-03-01H-Benzimidazole-5-carboxylicacid, 7-methyl-2-propyl-
- 1074-82-4Potassium phthalimide
- 920-66-12-Propanol,1,1,1,3,3,3-hexafluoro-
- 12054-85-2Ammonium molybdate tetrahydrate
- 627-30-53-Chloro-1-propanol
- 31430-15-6Flubendazole
- 105-45-3Butanoic acid, 3-oxo-,methyl ester
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Consensus Reports
NTP 10th Report on Carcinogens. IARC Cancer Review: Group 1 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 264.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 2 ,1973,p. 126.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 11 ,1976,p. 75.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Nickel and its compounds are on the Community Right-To-Know List. Reported in EPA TSCA Inventory.
Standards and Recommendations
OSHA PEL: TWA 1 mg(Ni)/m3
ACGIH TLV: TWA 1 mg(Ni)/m3; Not Classifiable as a Human Carcinogen
DFG MAK: DFG TRK: 0.5 mg/m3; Human Carcinogen
NIOSH REL: (Inorganic Nickel) TWA 0.015 mg(Ni)/m3
Analytical Methods
Nickel(II) oxide (CAS NO.1313-99-1),its occupational chemical analysis use NIOSH: Nitric Oxide s321.
Specification
The Nickel(II) oxide, with the cas registry number 1313-99-1, is a kind of green to dark green powder. This is soluble in acid and ammonia water while insoluble in water and liquid ammonia and it is stable chemically but incompatible with strong acids. When heated to 400 °C, it will produce nickelic trioxide after absorbing oxygen, and then restore to nickel monoxide when heated to 600 °C.
The product categories of this chemical are as follows: Inorganics; NickelNanomaterials; 28: Ni; Nanomaterials; Nanoparticles: Oxides, Nitrides, and Other CeramicsMetal and Ceramic Science; Nanopowders and Nanoparticle Dispersions; Electrode MaterialsMetal and Ceramic Science; NickelSupported Synthesis; Catalysis and Inorganic Chemistry; Chemical Synthesis; Reagents; Silica Gel; Alternative Energy; Electrode Materials; Materials Science; Metal and Ceramic Science; Oxides.
The characteristics of this chemical are as follows: (1)#H bond acceptors: 1; (2)#H bond donors: 0; (3)#Freely Rotating Bonds: 0; (4)Polar Surface Area: 17.07; (5)Exact Mass: 73.930263; (6)MonoIsotopic Mass: 73.930263; (7)Topological Polar Surface Area: 17.1; (8)Heavy Atom Count: 2; (9)Formal Charge: 0; (10)Complexity: 2.
As to its usage, it is widely applied in may ways. It could be used as adherence promotors and coloring agent of enamel, and also as the pigment of ceramics and glass; It could be also used in producing the chemical of nickel-zincferrite in magnetism material, nickel salt raw material, nickel (Ni) catalytic agent and also in application of metallurgy and kinescope; Then it could also be used as the electronic component materials.
People should be careful while dealing with this chemical. For one thing, it is toxic which could at low levels cause damage to health, and may cause cancer by inhalation. For another thing, it is irritant that may cause inflammation to the skin or other mucous membranes, and could cause sensitization by skin contact. In addition, it may cause long-term adverse effects in the aquatic environment. You should take the following instructions. Wear suitable protective clothing and gloves, and avoid exposure - obtain special instructions before use. If in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible). Avoid release to the environment, and you could efer to special instructions / safety data sheets.
You could obtain the molecular structure by converting the following datas:
(1)Canonical SMILES: O=[Ni]
(2)InChI: InChI=1S/Ni.O
(3)InChIKey: GNRSAWUEBMWBQH-UHFFFAOYSA-N
Below are the toxicity information of this chemical:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | intravenous | 12700ug/kg (12.7mg/kg) | Environmental Quality and Safety, Supplement. Vol. 1, Pg. 1, 1975. | |
| dog | LDLo | intravenous | 9mg/kg (9mg/kg) | Environmental Quality and Safety, Supplement. Vol. 1, Pg. 1, 1975. | |
| mouse | LD50 | subcutaneous | 50mg/kg (50mg/kg) | Zhurnal Vsesoyuznogo Khimicheskogo Obshchestva im. D.I. Mendeleeva. Journal of the D.I. Mendeleeva All-Union Chemical Society. Vol. 19, Pg. 186, 1974. | |
| rat | LDLo | intratracheal | 20mg/kg (20mg/kg) | National Technical Information Service. Vol. AEC-TR-6710, | |
| rat | LDLo | oral | 5gm/kg (5000mg/kg) | Food & Drug Research Laboratories, Inc., Papers. Vol. 7684B, Pg. 1983, |
-
Premium Related Products