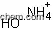Products Categories
| CAS No.: | 107-92-6 |
|---|---|
| Name: | n-Butanoic acid |
| Article Data: | 1027 |
| Molecular Structure: | |
|
|
|
| Formula: | C4H8O2 |
| Molecular Weight: | 88.1063 |
| Synonyms: | n-Butanoic acid;n-Butyric acid;(3R,4S)-1-Benzoyl-3-(1-methoxy-1-methylethoxy)-4-phenyl-2-azetidinone;Butyricacid (6CI,7CI,8CI);1-Propanecarboxylic acid;Ethylacetic acid;Honey robber;NSC 8415;Propylformic acid; |
| EINECS: | 203-532-3 |
| Density: | 0.987 g/cm3 |
| Melting Point: | -6 - -3 °C |
| Boiling Point: | 164.3 °C at 760 mmHg |
| Flash Point: | 69 °C |
| Solubility: | miscible with water |
| Appearance: | colourless liquid |
| Hazard Symbols: |
 Xi, Xi, C C
|
| Risk Codes: | 34 |
| Safety: | 26-36-45 |
| Transport Information: | UN 2820 8/PG 3 |
| PSA: | 37.30000 |
| LogP: | 0.87110 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 1219080-61-1IMIDAZOLE-2-BORONIC ACID

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrogen; [RhCl(Ph3P)2]; Ph2PO2CCH=CMe2 In acetone at 22℃; under 2280 Torr; for 17h; | 100% |
| With sodium tetrahydroborate; sodium hydroxide In water at 20 - 60℃; | 90% |
| With sodium hydroxide; hydrogen; nickel In water hydrogen generated in situ electrochemically on Raney nickel electrode; | 60% |

| Conditions | Yield |
|---|---|
| With 2,2,2-trichloroethylperoxycarbonic acid; dihydrogen peroxide In dichloromethane Ambient temperature; | 100% |
| With C4H11FeMo6NO24(3-)*3C16H36N(1+); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 8h; Green chemistry; | 99% |
| With 4H3N*4H(1+)*CuMo6O18(OH)6(4-); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate; sodium trimethylsilylpropionate-d4; C18H22N4O2Ru(2+)*2F6P(1-); water at 20℃; for 0.5h; | 100% |
| With oxygen In water at 80℃; under 760.051 Torr; for 24h; | 99.7% |
| With potassium hydroxide at 50℃; electrolysis; | 98.8% |
- 13022-83-8
2-oxopentanoic acid sodium salt

- 107-92-6
butyric acid

| Conditions | Yield |
|---|---|
| With calcium hypochlorite; acetic acid In dichloromethane; water; acetonitrile for 3h; Ambient temperature; | 96% |
- 199921-04-5
5-methyl-1-propyl-2,7,8-trioxabicyclo[3.2.1]octane

- 107-92-6
butyric acid

| Conditions | Yield |
|---|---|
| Stage #1: 5-methyl-1-propyl-2,7,8-trioxabicyclo[3.2.1]octane With pyridinium p-toluenesulfonate In methanol; water at 22℃; for 1.5h; Ring cleavage; Stage #2: With lithium hydroxide In tetrahydrofuran at 22℃; for 6h; Hydrolysis; | 96% |
- 3068-88-0, 32082-74-9, 36536-46-6, 65058-82-4
4-methyloxetan-2-one

- 107-92-6
butyric acid

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; W(OTf)6; hydrogen at 135℃; under 760.051 Torr; for 12h; | 96% |
| With palladium on activated carbon; W(OTf)6; hydrogen In neat (no solvent) at 135℃; under 760.051 Torr; for 12h; | 96% |
| With hydrogen at 200 - 247℃; under 15514.9 Torr; for 5.66667h; Inert atmosphere; | 94.2 %Chromat. |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; Na12[WZn3(H2O)2(ZnW9O34)2] at 75℃; for 7h; | A 95% B 4% |
| With Na12[WZn3(H2O)2(ZnW9O34)2]; dihydrogen peroxide at 85℃; for 7h; | |
| With SiW11Zn; dihydrogen peroxide In water at 89.85℃; for 9h; |
- 106-31-0
butanoic acid anhydride

- 691909-53-2
4-chloro-N-(2-fluoro-5-chlorophenyl)-N-(1R)-(2-hydroxy-1-methylpentyl)benzenesulfonamide

- 107-92-6
butyric acid

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane | 94% |

| Conditions | Yield |
|---|---|
| With benzyl(triethyl)ammoniumpermanganate In dichloromethane | A 93% B 5% |
- 539-86-6Allicin
- 4468-02-4Zinc gluconate
- 3734-33-6Denatonium benzoate
- 110-94-1Glutaric acid
- 7320-34-5Potassium pyrophosphate
- 83-86-3Phytic acid
- 108-55-42H-Pyran-2,6(3H)-dione,dihydro-
- 113507-06-5Moxidectin
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Specification
The IUPAC name of this chemical is Butyric acid. With the CAS registry number 107-92-6 and EINECS registry number 203-532-3, it is also named as ethylacetic acid. In addition, the molecular formula is C4H8O2. It is an oily colorless liquid that is easily soluble in water, ethanol, and ether, and can be separated from an aqueous phase by saturation with salts such as calcium chloride. And it has an unpleasant smell and acrid taste. Besides, it is found in butter, parmesan cheese, vomit, and as a product of anaerobic fermentation (including in the colon and as body odor).
Physical properties about this chemical are: (1)ACD/LogP: 0.70; (2)# of Rule of 5 Violations: 0; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 8.756; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 2; (8)#H bond donors: 1; (9)#Freely Rotating Bonds: 2; (10)Polar Surface Area: 37.3 Å2; (11)Index of Refraction: 1.411; (12)Molar Refractivity: 22.145 cm3; (13)Molar Volume: 89.188 cm3; (14)Polarizability: 8.779 ×10-24cm3; (15)Surface Tension: 32.584 dyne/cm; (16)Density: 0.988 g/cm3; (17)Flash Point: 69.712 °C; (18)Enthalpy of Vaporization: 42.395 kJ/mol; (19)Boiling Point: 164.348 °C at 760 mmHg; (20)Vapour Pressure: 1.351 mmHg at 25°C.
Preparation of Butyric acid: It is industrially prepared by the fermentation of sugar or starch, brought about by the addition of putrefying cheese, with calcium carbonate added to neutralize the acids formed in the process. In addition, it can be prepared by butan-1-ol. The other product is butyraldehyde. This reaction will need reagent NaBrO3 and catalyst Ru2(dmnapy)Cl4. The reaction time is 15 hours with ambient temperature. The yield is about 91.5%.
![]()
Uses of Butyric acid: it can be used as food, perfume and fishing bait additives, an animal feed supplement, an approved food flavouring. In addition, it is used in the preparation of various butyrate esters. Besides, it can react with 4-methyl-quinoline to get 4-methyl-2-propyl-quinoline. This reaction will need reagents sulfuric acid and iron(III) sulfate, and solvent H2O. The reaction time is 20 hours with irradiation. The yield is about 76%.

When you are using this chemical, please be cautious about it as the following:
This chemical can cause burns. During using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.).
You can still convert the following datas into molecular structure:
(1)SMILES: CCCC(=O)O
(2)InChI: InChI=1/C4H8O2/c1-2-3-4(5)6/h2-3H2,1H3,(H,5,6)
(3)InChIKey: FERIUCNNQQJTOY-UHFFFAOYAP
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC | inhalation | > 500mg/m3 (500mg/m3) | LUNGS, THORAX, OR RESPIRATION: STRUCTURAL OR FUNCTIONAL CHANGE IN TRACHEA OR BRONCHI | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 23(5), Pg. 31, 1958. |
| mouse | LD50 | intraperitoneal | 3180mg/kg (3180mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 21, Pg. 85, 1969. | |
| mouse | LD50 | intravenous | 800mg/kg (800mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Acta Pharmacologica et Toxicologica. Vol. 18, Pg. 141, 1961. |
| mouse | LD50 | subcutaneous | 3180mg/kg (3180mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 21, Pg. 85, 1969. | |
| mouse | LDLo | oral | 500mg/kg (500mg/kg) | GASTROINTESTINAL: NECROTIC GHANGES KIDNEY, URETER, AND BLADDER: OTHER CHANGES BLOOD: CHANGES IN SPLEEN | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 4, Pg. 19, 1962. |
| rabbit | LD50 | skin | 530uL/kg (0.53mL/kg) | Union Carbide Data Sheet. Vol. 4/10/1968, | |
| rat | LC | inhalation | > 500mg/m3 (500mg/m3) | LUNGS, THORAX, OR RESPIRATION: STRUCTURAL OR FUNCTIONAL CHANGE IN TRACHEA OR BRONCHI | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 4, Pg. 19, 1962. |
| rat | LD50 | oral | 2gm/kg (2000mg/kg) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 30, 1982. |