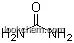Products Categories
| CAS No.: | 97-59-6 |
|---|---|
| Name: | Allantoin |
| Article Data: | 114 |
| Molecular Structure: | |
|
|
|
| Formula: | C4H6N4O3 |
| Molecular Weight: | 158.117 |
| Synonyms: | (2,5-dioxoimidazolidin-4-yl)urea;Glyoxylic(acid) diureide;urea, N-(2,5-dioxo-4-imidazolidinyl)-;Cutemol emollient;[(4S)-2,5-dioxoimidazolidin-4-yl]urea;5-Ureido-2,4-imidazolidindion;[(4R)-2,5-dioxoimidazolidin-4-yl]urea;Urea, (2,5-dioxo-4-imidazolidinyl)-;Urea, (2,5-dioxo-4-imidazolidinyl)- (9CI);Glyoxyldiureide;Urea, (2, 5-dioxo-4-imidazolidinyl)-;Glyoxylic diureide;(2,5-Dioxo-4-imidazolidinyl)urea;5-Ureidohydantoin;Allantoin (5-Ureidohydantoin);Urea,(2,5-dioxo-4-imidazolidinyl)-; |
| EINECS: | 202-592-8 |
| Density: | 1.652 g/cm3 |
| Melting Point: | 230 °C (dec.)(lit.) |
| Boiling Point: | 478oC |
| Flash Point: | 230-234 °C |
| Solubility: | Slightly soluble in water. Freely soluble in alkalis |
| Appearance: | White crystalline powder |
| Hazard Symbols: |
 Xi, Xi, Xn Xn
|
| Risk Codes: | 22-36/37/38 |
| Safety: | 22-24/25-36-26 |
| PSA: | 113.32000 |
| LogP: | -0.43100 |
- 99170-93-1N-Methyl-2-oxazolamine
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile
- 98020-47-46-Amino-5-nitro-2-thio-uracil
- 98025-62-8(5-METHYL-[1,3,4]THIADIAZOL-2-YL)-HYDRAZINE
- 99724-17-1Dimethyl-pyrrolidin-3-ylmethyl-amine
- 98084-25-4CESIUM TITANATE 99.9+%
- 98296-48-1GANODERIC ACID C2(SH)
- 99421-19-9ethyl 3-amino-6-chloro-5-cyano-2-methylisonicotinate
- 99689-20-02-Acetamido-3-O-(D-1-carboxyethyl)-2-deoxy-2-D-glucose Methyl Ester
- 98730-77-91-(hydroxymethyl)cyclopropanecarbonitrile

| Conditions | Yield |
|---|---|
| With Co[PyPS]2Mo11VO40 at 90℃; for 10h; Temperature; Reagent/catalyst; Microwave irradiation; | 89.3% |
| With titanium dioxide-based composite solid catalyst A1 at 75℃; for 4h; | 85% |
| at 60℃; for 1.5h; Concentration; | 82.4% |
- 69-93-2
uric Acid

A
- 120-89-8
parabanic acid

B
- 5676-27-7
Oxalyldiurea

C
- 105245-87-2
dehydro-allantoin

D
- 97-59-6
Allantoin

| Conditions | Yield |
|---|---|
| With lithium hydroxide; iodine In water for 0.0833333h; excess of I2; | A n/a B n/a C 75% D n/a |
| With lithium hydroxide; iodine In water for 0.0833333h; Mechanism; also with substituted uric acid; effect of amount of I2; 2H and 13C labelling experiment; |


| Conditions | Yield |
|---|---|
| With lithium hydroxide; iodine In water at 4℃; equimolar amount of I2; | 70% |
| With sodium hydroxide; oxygen; pyrographite | |
| With water; ozone |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium permanganate In water for 4h; Product distribution; Mechanism; Ambient temperature; labelled 14C in position of 5; | A 26% B 14% |

| Conditions | Yield |
|---|---|
| In nitromethane for 1h; Reflux; | 15% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium permanganate In water for 4h; Ambient temperature; Yield given; | A n/a B 14% |

| Conditions | Yield |
|---|---|
| With bromine; acetic acid |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; copper dichloride |

| Conditions | Yield |
|---|---|
| With potassium nitrite; acetic acid |

| Conditions | Yield |
|---|---|
| With water |
- 51-67-2Tyramine
- 98-59-9Benzenesulfonylchloride, 4-methyl-
- 1134-47-0Baclofen
- 91-64-5Coumarin
- 33419-42-0Etoposide
- 144689-93-01H-Imidazole-5-carboxylicacid, 4-(1-hydroxy-1-methylethyl)-2-propyl-, ethyl ester
- 26172-55-45-Chloro-2-methyl-4-isothiazolin-3-one
- 124-41-4Sodium methanolate
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Specification
The Allantoin, with the CAS registry number 97-59-6, is also known as 1-(2,5-Dioxo-4-imidazolidinyl)urea. It belongs to the product categories of Substrates; Intermediates & Fine Chemicals; Metabolites & Impurities; Pharmaceuticals. Its EINECS registry number is 202-592-8. This chemical's molecular formula is C4H6N4O3 and molecular weight is 158.12. What's more, its IUPAC name is called (2,5-Dioxoimidazolidin-4-yl)urea. It should be stored in a cool, dry and well-ventilated place. In fish, Allantoin is broken down further (into ammonia) before excretion. It is a major metabolic intermediate in many other organisms including plants and bacteria. It is frequently present in toothpaste, mouthwash, and other oral hygiene products, in shampoos, lipsticks, anti-acne products, sun care products, and clarifying lotions, various cosmetic lotions and creams, and other cosmetic and pharmaceutical products.
Physical properties about Allantoin are: (1)ACD/LogP: -1.523; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): -1.52; (4)ACD/LogD (pH 7.4): -1.62; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 3.53; (8)ACD/KOC (pH 7.4): 2.81; (9)#H bond acceptors: 7; (10)#H bond donors: 5; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 113.32 Å2; (13)Index of Refraction: 1.616; (14)Molar Refractivity: 33.422 cm3; (15)Molar Volume: 95.701 cm3; (16)Polarizability: 13.25×10-24cm3; (17)Surface Tension: 82.625 dyne/cm; (18)Density: 1.652 g/cm3.
Preparation of Allantoin: this chemical can be prepared by 7,9-dihydro-3H-purine-2,6,8-trione. This reaction needs reagents I2, LiOH and solvent H2O at temperature of 4 °C. The yield is 70 %.

Uses of Allantoin: it is used to produce other chemicals. For example, it can react with 1-(3-iodo-propyl)-3,7-dimethyl-3,7-dihydro-purine-2,6-dione to get {1-[3-(3,7-dimethyl-2,6-dioxo-2,3,6,7-tetrahydro-purin-1-yl)-propyl]-2,5-dioxo-imidazolidin-4-yl}-urea. The reaction occurs with reagent NaOMe and other condition of heating for 1.5 hours. The yield is 40 %.
![Allantoin can react with 1-(3-iodo-propyl)-3,7-dimethyl-3,7-dihydro-purine-2,6-dione to get {1-[3-(3,7-dimethyl-2,6-dioxo-2,3,6,7-tetrahydro-purin-1-yl)-propyl]-2,5-dioxo-imidazolidin-4-yl}-urea.](/UserFilesUpload/Uses of Allantoin.jpg)
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. It has serious damage to eyes. If swallowed, it's harmful to health. Therefore, you should wear suitable protective clothing. The gas can not be breathed and you should avoid contacting with skin and eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: C1(C(=O)NC(=O)N1)NC(=O)N
(2) InChI: InChI=1S/C4H6N4O3/c5-3(10)6-1-2(9)8-4(11)7-1/h1H,(H3,5,6,10)(H2,7,8,9,11)
(3) InChIKey: POJWUDADGALRAB-UHFFFAOYSA-N