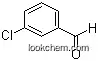
587-04-2Relevant articles and documents
Hydrothermal self - sacrificing growth of polymorphous MnO2 on magnetic porous - carbon (Fe3O4@Cg/MnO2): A sustainable nanostructured catalyst for activation of molecular oxygen
Bakhtiarzadeh, Zohreh,Jang, Ho Won,Karimi, Ziba,Kim, Dokyoon,Msagati, Titus A. M.,Ramakrishna, Seeram,Rostamnia, Sadegh,Rouhani, Shamila,Shokouhimehr, Mohammadreza,Varma, Rajender S.
, (2021)
Novel core-shell carbon coated-magnetic (Fe3O4@Cg) nanoparticles supported MnO2 nanosheets (with α- and β-type structure) (Fe3O4@Cg/MnO2) are synthesized through a self-sacrificing templet method. The new hybrid material was fully characterized with Fourier transformed infrared spectroscopy, energy-dispersive X-ray spectroscopy, scanning electron microscopy, X-ray diffraction analysis (XRD), N2 adsorption/desorption analysis, and transmission electron microscopy; XRD and SEM results affirmed that α- and β-MnO2 nanosheets polymorphs onto the Fe3O4@Cg. The catalytic activity of the as-prepared nanostructured catalyst Fe3O4@Cg/MnO2 has been evaluated in O2 activation for the selective oxidation of benzyl alcohol to benzaldehyde with high conversion; it stability being confirmed by the recycling of the nanostructured catalyst with no obvious loss even after six repeated runs.
Boosted photocatalytic performance of uniform hetero-nanostructures of Bi2WO6/CdS and Bi2WO6/ZnS for aerobic selective alcohol oxidation
Safaei, Elham,Mohebbi, Sajjad
, p. 173 - 181 (2019)
A two-dimensional hierarchical structural photocatalyst based on Bi2WO6 was obtained via a hydrothermal method and modified using ZnS and CdS and characterized by physico-chemical techniques XRD, FTIR, SEM, EDAX, and DRS. Their photo
Controlled reduction of activated primary and secondary amides into aldehydes with diisobutylaluminum hydride
Azeez, Sadaf,Kandasamy, Jeyakumar,Sabiah, Shahulhameed,Sureshbabu, Popuri
supporting information, p. 2048 - 2053 (2022/03/31)
A practical method is disclosed for the reduction of activated primary and secondary amides into aldehydes using diisobutylaluminum hydride (DIBAL-H) in toluene. A wide range of aryl and alkyl N-Boc, N,N-diBoc and N-tosyl amides were converted into the corresponding aldehydes in good to excellent yields. Reduction susceptible functional groups such as nitro, cyano, alkene and alkyne groups were found to be stable. Broad substrate scope, functional group compatibility and quick conversions are the salient features of this methodology.
SBA-15 Supported Silver Catalyst for the Efficient Aerobic Oxidation of Toluene Under Solvent-Free Conditions
Chen, Lei,Chen, Yanjiao,Dai, Xuan,Guo, Jiaming,Peng, Xinhua
, (2021/12/09)
The efficient SBA-15 supported silver catalysts(Ag/SBA-15) were prepared and characterized by ICP-OES, XRD, TEM, SEM, XPS and N2 adsorption–desorption techniques. The catalysts exhibited an excellent catalytic activity for the aerobic oxidation of toluene to benzaldehyde under solvent-free conditions. Conversion of toluene and selectivity of benzaldehyde were 50% and 89% respectively over catalyst with 9.1 wt% Ag loading (10Ag/SBA-15). A wide range of substrates were tolerated under the selected reaction conditions. The kinetic study shows that the oxidation of toluene over 10Ag/SBA-15 is pseudo-first-order reaction and the activation energy Ea is 45.1?kJ/mol. A plausible mechanism involving oxygen free radicals was proposed for the aerobic oxidation reaction. Compared with the traditional method, the newly designed heterogeneous catalytic system shows better economic applicability, environmental friendliness and broader application prospects. Graphical abstract: [Figure not available: see fulltext.]
Rapid, chemoselective and mild oxidation protocol for alcohols and ethers with recyclable N-chloro-N-(phenylsulfonyl)benzenesulfonamide
Badani, Purav,Chaturbhuj, Ganesh,Ganwir, Prerna,Misal, Balu,Palav, Amey
supporting information, (2021/06/03)
Chlorine is the 20th most abundant element on the earth compared to bromine, iodine, and fluorine, a sulfonimide reagent, N-chloro-N-(phenylsulfonyl)benzenesulfonamide (NCBSI) was identified as a mild and selective oxidant. Without activation, the reagent was proved to oxidize primary and secondary alcohols as well as their symmetrical and mixed ethers to corresponding aldehydes and ketones. With recoverable PS-TEMPO catalyst, selective oxidation over chlorination of primary and secondary alcohols and their ethers with electron-donating substituents was achieved. The reagent precursor of NCBSI was recovered quantitatively and can be reused for synthesizing NCBSI.
A new porous Co(ii)-metal-organic framework for high sorption selectivity and affinity to CO2and efficient catalytic oxidation of benzyl alcohols to benzaldehydes
Wu, Yun-Long,Yang, Rong-Rong,Yang, Guo-Ping,Yan, Yang-Tian,Su, Xiao-Lei,He, Xin-Hai,Song, Yan-Yan,Ma, Zheng-Sheng,Wang, Yao-Yu
, p. 3717 - 3723 (2021/05/31)
Herein, we report a new 3D porous Co(ii)-based metal-organic framework catalyst (Me2NH2)2[Co3(L)2(H2O)2]·2DMF (MOF I), which has been successfully prepared by using Co(ii) ions and rigid V-shaped 3,5-di(2,4-dicarboxylphenyl)pyridine (H4L) via the solvothermal reaction. Structural analysis reveals that I displays a porous structure with the pore size of 16.2 × 7.2 ?2 based on the trinuclear [Co3(COO)4(H2O)2N2] secondary building units (SBUs). Gas sorption experiments on the guest free sample I′ reveals a high capacity and selectivity to CO2 over CH4. And further, the catalytic explorations of the I′-catalyzed system (I′: 3 mol%; proline: 40 mol%; CH3CN: 2 mL) reveal that benzyl alcohols with different structures can be efficiently transformed into benzyl alcohols without by-products under mild conditions.
Fe(III) superoxide radicals in halloysite nanotubes for visible-light-assisted benzyl alcohol oxidation and oxidative C[sbnd]C coupling of 2-naphthol
Bania, Kusum K.,Baruah, Manash J.,Bora, Tonmoy J.,Dutta, Rupjyoti,Guha, Ankur Kanti,Roy, Subhasish
, (2021/09/20)
Selective oxidation of benzyl alcohols to aldehydes and 2-naphthol to BINOL was achieved by activation of molecular oxygen (O2) and hydrogen peroxide (H2O2) over an iron-oxide catalyst embedded in halloysite nanotube. Electron spin resonance spectroscopy (ESR), Raman and in situ FTIR spectroscopic analysis provided direct evidence for the involvement of superoxide radical bound FeIII species in the oxidation reaction. Both the analysis suggested the end-on binding of superoxide radical with FeIII-centre. The stability of such radical bound FeIII-species in halloysite nanotube was analyzed through density functional theory (DFT) calculations. Results suggested that end-on (η1) binding was favourable by 13.5 kcal/ mol than the side-on (η2) binding mode. The formation of such reactive species was believed to play the crucial role in bringing the high selectivity in the catalytic oxidation of benzyl alcohol and oxidative C[sbnd]C coupling of 2-naphthol. UV–Vis spectroscopic studies on the oxidation of benzyl alcohol suggested for the initial adsorption of substrate molecule on the catalyst surface followed by its interaction with FeIII -superoxide/hydroperoxide species generated upon photoirradiation with visible light in presence of O2. The presence of a suitable band gap ~2.14 eV enabled the catalyst to catalyze the reaction under visible light irradiation. Both the reactions (benzyl alcohol and 2-naphthol oxidation) were tested in presence of both O2 and H2O2 as oxidants at ambient temperature. The influence of different parameters like rate of oxygen flow, amount of peroxide, nature of solvent, and catalyst amount on the conversion and selectivity of the reactions were studied to understand their role in the catalytic reactions. Successful oxidation of 2-naphthol with H2O2 as oxidant was a real success to overcome the limitations associated with this reaction using H2O2 as oxidant.
α-MnO2 modified exfoliated porous g-C3N4 nanosheet (2D) for enhanced photocatalytic oxidation efficiency of aromatic alcohols
Nanda, Binita,Nanda, Braja B.,Pradhan, Manas Ranjan,Rath, Dharitri,Sethi, Ratikanta
, (2021/06/18)
Porous graphitic carbon nitride (g-C3N4) was synthesized by taking melamine and ammonium bicarbonate through single-step calcination method followed by ultrasonication to obtain exfoliated porous g-C3N4 (2D) nanosheets. Further enhancement of photocatalytic performance, g-C3N4 nanosheet (2D) was further modified with different weight percentage of (1, 3, 5, and 7) of MnO2. The introduction of α-MnO2 onto the g-C3N4 nanosheet establishes an interlayer channels to promote the migration of charge carriers through the valence band and conduction band of the prepared composite MnO2@g-C3N4. The transformation of photo induced charge carriers adopt the Z-scheme mechanism rather band-transfer mechanism. The accumulated photo generated electrons in conduction band of g-C3N4 is more electro negative than the potential of (O2/O2–.) and able to reduce oxygen to superoxide (O2–.) radical. At the same time, the holes in valence band of α-MnO2 are more electro positive than the potential of (OH–/OH.) and help in oxidate OH– to hydroxyl (OH.) radical. Among all the composites, 3 wt% MnO2 modified g-C3N4 shows the best photocatalytic oxidation efficiency towards all the aromatic alcohols. In presence of visible light, heterojuction formation, and formation of active charged species (OH. and O2–.) were mostly responsible for photocatalytic oxidation of aromatic alcohols through free radical mechanism.
Synthesis, characterization, reactivity, and catalytic studies of heterobimetallic vanadium(V) complexes containing hydrazone ligands
Borthakur, Rosmita,Dhanpat, Shobha A.,Kumar, Arvind,Kurbah, Sunshine D.,Lal, Ram A.,Syiemlieh, Ibanphylla
, (2020/10/21)
Six heterobimetallic alkali metal dioxidovanadium(V) coordination polymer complexes {[M6{VO(μ-O)}2(μ-OH)4(μ4-slox/nph)].n DMF}∞ where M = Na, K, and Cs; n = 1 for (1), 0 for (2)-(6) of two dihydrazone ligands, disalicylaldehydeoxaloyldihydrazone (H4slox) and bis(2-hydroxy-1-naphthaldehyde)oxaloyldihydrazone (H4nph) are reported. All the complexes have been characterized by various physicochemical techniques such as elemental analyses, molar conductance, IR, NMR, UV–vis, and cyclic voltammetry. The IR, 1HNMR, and 13CNMR spectral data suggest that the dihydrazones are coordinated through phenolate/naphtholate oxygen, enolate oxygen, and azine nitrogen atoms to the metal centres. The structure of complex {[Na6{VO(μ-O)}2(μ-OH)4(μ4-slox)].DMF}∞ (1) is also determined by single-crystal X-ray data, which revealed that the H4slox coordinated via all possible dative sites to metal centres as tetrabasic octadentate ligand. The vanadium metal centres adopted distorted square-pyramidal coordination geometries, and the sodium atoms are also in five coordination atmospheres. The electronic spectra of the complexes showed LMCT bands in addition to intra-ligand π → π* and n → π* transitions. As evident from the cyclic voltammetry, the complexes showed two metal-centred electron transfer reactions {[(VVVV(slox)2?/VVVIV(slox)3?] and [(VVVIV(slox)3?/VVVIV(slox)4?]}, in addition to the ligand centred electron transfer reactions. Further, bovine serum albumin (BSA interaction studies of the complexes {[Na6{VO(μ-O)}2(μ-OH)4(μ4-slox)].DMF}∞ (1) and [Na6{VO(μ-O)}2(μ-OH)4(μ4-nph)]∞ (4) revealed strong binding affinity. Moreover, the catalytic studies of the complexes (1) and (4) were found to be effective for the oxidation of alcohols into their corresponding aldehydes and ketones and bromination of some organic substrates in the presence of H2O2 as an oxidizing agent.
An expeditious and efficient method for the oxidation of benzyl alcohols by homogeneous electrolysis
Jagatheesan, Rathinavel,Shanmugavelan, Poovan,Sambathkumar, Subramaniyan,Ramesh, Pugalenthi
supporting information, p. 3013 - 3022 (2021/08/12)
A greener and inexpensive electrochemical method has been developed for the oxidation of benzyl alcohols by homogeneous electrolysis. The electrochemical reaction was carried out in an undivided cell equipped with carbon and stainless steel electrodes at room temperature. The homogeneous solution made up of acetonitrile/water containing substrate and ammonium bromide with a catalytic amount of H2SO4 as supporting electrolyte. The reaction condition was optimized with various electrochemical experimental parameters and evaluated with various substituted benzyl alcohols to result in excellent yield of aldehydes (>83%).