- 7545-71-3Phenol,2-(1-methylethyl)-6-nitro-
- 75462-59-83,5-Bis(trifluoromethyl)benzyl chloride
- 75462-61-2Benzene,1-methyl-3,5-bis(trifluoromethyl)-
- 7546-27-2Benzenesulfonic acid,3-hydroxy-4-methoxy-, calcium salt (2:1)
- 75464-92-55-Pyrimidinecarboxylicacid, 4-amino-2-[(2,4-dinitrophenyl)thio]-, ethyl ester
- 75-46-7Methane, trifluoro-
- 75467-36-6D-Ribonic acid,5-O-[(1,1-dimethylethyl)dimethylsilyl]-2,3-O-(1-methylethylidene)-, g-lactone
- 754-67-61-Penten-3-ol,4,4,5,5,5-pentafluoro-3-methyl-
- 75472-91-21,5-Benzothiazepin-4(5H)-one,5-[2-(dimethylamino)ethyl]-2,3-dihydro-3-hydroxy-2-(4-methoxyphenyl)-,hydrochloride (1:1), (2S,3S)-
- 75473-09-5Benzenepropanenitrile, 2,3-dichloro-b-oxo-
Hot Products
- 5308-25-81-Ethylpiperazine
- 793-24-81,4-Benzenediamine,N1-(1,3-dimethylbutyl)-N4-phenyl-
- 333-20-0Potassium thiocyanate
- 14047-28-09H-Purine-9-ethanol,6-amino-a-methyl-, (aR)-
- 110-65-62-Butyne-1,4-diol
- 5535-48-8Phenyl vinyl sulfone
- 104632-26-0Pramipexole
- 34160-40-22-Pyridinecarboxaldehyde,6-bromo-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
Difluorochloromethane |
EINECS | 200-871-9 |
| CAS No. | 75-45-6 | Density | 1.277 g/cm3 |
| PSA | 0.00000 | LogP | 1.44780 |
| Solubility | slightly soluble in water | Melting Point |
-146 °C |
| Formula | CHClF2 | Boiling Point | -40.8 °C |
| Molecular Weight | 86.4687 | Flash Point | N/A |
| Transport Information | UN 1018 | Appearance | Colourless gas |
| Safety | 23-59 | Risk Codes | 40-59 |
| Molecular Structure |
|
Hazard Symbols |
 F F
|
| Synonyms |
Algeon 22;Algofrene 22;Algofrene 6;Arcton 22;Arcton 4;CFC 22;Chlorodifluoromethane;Daiflon 22;Difluoromonochloromethane;Dymel 22;F 22 (halocarbon);FC 22;FC 22 (halocarbon);FKW 22;Flugene22;Forane 22;Freon 22;Frigen 22;Fron 22;HFA 22;Haltron 22;Isceon 22;Isotron 22;Khladon 22;Korfron 22;Propellant 22;Refrigerant R 22;Solkane 22;Ucon 22; |
Article Data | 92 |
Difluorochloromethane Synthetic route

- 75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| With 2,2,2-trifluoroethanol In N,N,N,N,N,N-hexamethylphosphoric triamide decompn. in the presence of CF3CH2OH at reflux temp., 12 h; | 100% |
| In N,N,N,N,N,N-hexamethylphosphoric triamide |

| Conditions | Yield |
|---|---|
| nickel In neat (no solvent) steady-state flow reaction over Ni catalyst at 723 K; | A 98.3% B 0.6% |
| nickel In neat (no solvent) steady-state flow reaction over Ni catalyst at 673 K; | A 75.4% B 1.7% |
- 79-53-8
chloropentafluoroacetone

A
- 75-45-6
Chlorodifluoromethane

B
- 354-34-7
trifluoroacetyl fluoride

C
- 2925-22-6
2,2-difluoroacetyl fluoride

D
- 152239-89-9
C3HClF6O

| Conditions | Yield |
|---|---|
| With chlorine monofluoride; cesium fluoride 1.) -140 degC to -75 degC over 6 h, 2.) -75 degC, 2 h; Further byproducts given; | A n/a B n/a C n/a D 90% |
- 75-09-2
dichloromethane

- 7664-41-7
ammonia

A
- 74-90-8
hydrogen cyanide

B
- 75-10-5
Difluoromethane

C
- 75-45-6
Chlorodifluoromethane

D
- 124-38-9
carbon dioxide

E
- 201230-82-2
carbon monoxide

| Conditions | Yield |
|---|---|
| nickel titanate In neat (no solvent) steady-state flow reaction over NiTO3*6H2O catalyst at 773 K, further by-product is CFH3 (yield 0.3 %); | A 81.8% B 0.1% C 0.6% D 10.4% E 6.8% |
| nickel titanate In neat (no solvent) steady-state flow reaction over NiTO3*6H2O catalyst at 823 K, further by-product is CFH3 (yield 0.4 %); | A 66.2% B 0.2% C 1.4% D 12.9% E 18.8% |
| With catalyst: Pt/C In neat (no solvent) steady-state flow reaction over Pt/C catalyst at 821 K, a further by-product is CFH3 (yield 0.2 %); | A 64.6% B 0.3% C 0.2% D 0.6% E 0.8% |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; | 68% |
- 75-09-2
dichloromethane

- 7664-41-7
ammonia

A
- 74-90-8
hydrogen cyanide

B
- 593-53-3
Methyl fluoride

C
- 75-10-5
Difluoromethane

D
- 75-45-6
Chlorodifluoromethane

E
- 124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| With catalyst: Pt/C In neat (no solvent) steady-state flow reaction over Pt/C catalyst at 769 K; | A 53.4% B 0.3% C 0.5% D 7.6% E 0.5% |

- 1717-59-5
2,2-difluoro-2-(fluorosulfonyl)acetic acid

- 1005-30-7
sodium 4-iodo-benzoate

A
- 75-45-6
Chlorodifluoromethane

B
- 120608-87-9
4-Iodo-benzoic acid difluoromethyl ester

| Conditions | Yield |
|---|---|
| With potassium chloride In acetonitrile at 50℃; for 2.5h; | A n/a B 45% |
- 79-38-9
Chlorotrifluoroethylene

- 152239-89-9
C3HClF6O

A
- 75-45-6
Chlorodifluoromethane

B
- 354-34-7
trifluoroacetyl fluoride

C
- 2925-22-6
2,2-difluoroacetyl fluoride

| Conditions | Yield |
|---|---|
| In trichlorofluoromethane for 18h; -135 degC to 25 degC; Further byproducts given; | A n/a B n/a C n/a D 40% |


| Conditions | Yield |
|---|---|
| In ethanol decompn. with alc. KOH at 35°C in 48h;; | A 24% B n/a |
| In ethanol |
- 75-43-4
Dichlorofluoromethane

- 1188-14-3
Triethylgerman

A
- 75-10-5
Difluoromethane

B
- 75-09-2
dichloromethane

C
- 75-46-7
trifluoromethan

D
- 75-45-6
Chlorodifluoromethane

E
- 994-28-5
triethylchlorogermane

| Conditions | Yield |
|---|---|
| With ACF. Et3GeH at 25℃; for 72h; Schlenk technique; Inert atmosphere; | A n/a B n/a C 22% D 16% E n/a |

Difluorochloromethane Chemical Properties
Molecular Formula: CHClF2
Molecular Weight: 86.47
EINECS: 200-871-9
Index of Refraction: 1.278
Density: 1.277 g/cm3
Melting point: -146 °C
Boiling Point: 155 °C
Water solubility: Slightly soluble
Appearance: Colourless gas
Structure of Difluorochloromethane (CAS NO.75-45-6):
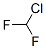
IUPAC Name: Chloro(difluoro)methane
Product Category of Difluorochloromethane (CAS NO.75-45-6): refrigerants;Organics;Refrigerant
Difluorochloromethane Uses
Difluorochloromethane (CAS NO.75-45-6) is mainly used as mainlyPropellant, fumigant, insecticide. It was once commonly used as a propellant and in air conditioning applications.
Difluorochloromethane Production
Difluorochloromethane (CAS NO.75-45-6) can be prepared from Chloroform : HCCl3 + 2HF → HCF2Cl + 2HCl .
Difluorochloromethane Toxicity Data With Reference
| 1. | mmo-sat 33 pph/24H-C | TOLED5 Toxicology Letters. 2 (1978),1. | ||
| 2. | mma-sat 33 pph/24H-C | TOLED5 Toxicology Letters. 2 (1978),1. | ||
| 3. | ihl-rat LC50:35 pph/15M | HUTODJ Human Toxicology. 1 (1982),239. | ||
| 4. | ihl-mus LC50:28 pph/20M | TXAPA9 Toxicology and Applied Pharmacology. 59 (1981),64. | ||
| 5. | ihl-dog LCLo:70 pph | TXAPA9 Toxicology and Applied Pharmacology. 2 (1960),363. |
Difluorochloromethane Consensus Reports
It is reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Difluorochloromethane Safety Profile
Hazard Codes:  F
F
Risk Statements: 40-59
R40:Limited evidence of a carcinogenic effect.
R59:Dangerous to the ozone layer.
Safety Statements: 23-59
S23:Do not breathe vapour.
S59:Refer to manufacturer / supplier for information on recovery / recycling.
Mildly toxic by inhalation. Experimental reproductive effects. Mutation data reported. An asphyxiant in high concentrations. At elevated pressures, 50% mixtures with air are combustible although ignition is difficult. When heated to decomposition it emits toxic fumes of F− and Cl−.
Difluorochloromethane Standards and Recommendations
OSHA PEL: TWA 1000 ppm
ACGIH TLV: TWA 1000 ppm; Not Classifiable as a Human Carcinogen
DFG MAK: 500 ppm (1800 mg/m3)
DOT Classification: 2.2; Label: Nonflammable Gas
Difluorochloromethane Specification
Difluorochloromethane , its cas register number is 75-45-6. It also can be called Chlorodifluoromethane ; Fluorocarbon 22 ; Freon R-22 ; and Freon 22 . Difluorochloromethane (CAS NO.75-45-6) is shipped as a liquefied gas under its own vapor pressure, and is noncombustible. Difluorochloromethane can asphyxiate by the displacement of air. Contact with the liquid can cause frostbite.

