Products Categories
| CAS No.: | 124-07-2 |
|---|---|
| Name: | Octanoic acid |
| Article Data: | 515 |
| Molecular Structure: | |
|
|
|
| Formula: | C8H16O2 |
| Molecular Weight: | 144.214 |
| Synonyms: | 1-Heptanecarboxylic acid;n-Caprylic acid;n-Octanoic acid;n-Octylic acid; |
| EINECS: | 204-677-5 |
| Density: | 0.929 g/cm3 |
| Melting Point: | 16 °C |
| Boiling Point: | 239.33 °C at 760 mmHg |
| Flash Point: | 107.379 °C |
| Solubility: | 0.68 g/L (20 ºC) |
| Appearance: | colourless oily liquid |
| Hazard Symbols: |
 C, C, Xi Xi
|
| Risk Codes: | 34 |
| Safety: | 26-36/39-45-36/37/39-25-27 |
| Transport Information: | UN 3265 8/PG 3 |
| PSA: | 37.30000 |
| LogP: | 2.43150 |
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; manganese; C36H40N2 In N,N-dimethyl-formamide at 25℃; under 760.051 Torr; for 20h; regioselective reaction; | 92% |
| With rubidium carbonate; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; (6,6’-dimethyl-2,2'-bipyridine)nickel(II) dibromide; tetra-(n-butyl)ammonium iodide In N,N-dimethyl-formamide at 10℃; under 750.075 Torr; for 20h; Schlenk technique; Sealed tube; Irradiation; | 48% |
| Stage #1: 2-bromoheptane; carbon dioxide With nickel(II) bromide dimethoxyethane; C32H32N2; C42H34F10IrN4(1+)*F6P(1-); N-ethyl-N,N-diisopropylamine; lithium tert-butoxide In N,N-dimethyl-formamide at 30℃; for 24h; Microwave irradiation; Schlenk technique; Stage #2: With hydrogenchloride In water; N,N-dimethyl-formamide Reagent/catalyst; | 25% |
| Stage #1: carbon dioxide With nickel(II) iodide; manganese; C36H40N2 In N,N-dimethyl-formamide at 25℃; under 760.051 Torr; Schlenk technique; Stage #2: 2-bromoheptane In N,N-dimethyl-formamide at 25℃; under 760.051 Torr; for 20h; Schlenk technique; Stage #3: With hydrogenchloride In water; N,N-dimethyl-formamide Reagent/catalyst; | 76 %Spectr. |

| Conditions | Yield |
|---|---|
| With nitric acid for 0.333333h; Ambient temperature; sonication; | 100% |
| With nitric acid for 0.333333h; Ambient temperature; sonication; | 100% |
| With ruthenium trichloride; iodobenzene; potassium peroxomonosulfate In water; acetonitrile at 20℃; for 16h; | 100% |

| Conditions | Yield |
|---|---|
| With tris[2-(4,6-difluorophenyl)pyridinato-C2,N]-iridium(III); oxygen In acetonitrile at 20℃; Irradiation; Sealed tube; Green chemistry; chemoselective reaction; | 99% |
| With diphenyl diselenide; dihydrogen peroxide In water at 20℃; for 3h; Green chemistry; | 99% |
| With copper acetylacetonate; oxygen; sodium hydroxide; 1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene In water at 50℃; under 760.051 Torr; for 12h; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; manganese; C36H40N2 In N,N-dimethyl-formamide at 25℃; under 760.051 Torr; for 20h; regioselective reaction; | 81% |
- 1974-04-5
2-bromoheptane

- 124-38-9
carbon dioxide

A
- 116454-37-6, 128441-06-5, 1188-02-9
2-methylheptanoic acid

B
- 124-07-2
Octanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromoheptane; carbon dioxide With nickel(II) iodide; manganese; 2,9-diethyl-4,7-diphenyl-1,10-phenanthroline In N,N-dimethyl-formamide at 30℃; under 760.051 Torr; for 17h; Schlenk technique; Stage #2: With hydrogenchloride In water; N,N-dimethyl-formamide Reagent/catalyst; Temperature; Solvent; | A n/a B 72% |
| With rubidium carbonate; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; (6,6’-dimethyl-2,2'-bipyridine)nickel(II) dibromide In N,N-dimethyl-formamide at 10℃; under 750.075 Torr; for 20h; Reagent/catalyst; Schlenk technique; Sealed tube; Irradiation; Overall yield = 48 %; Overall yield = 17.3 mg; |


| Conditions | Yield |
|---|---|
| With nickel(II) iodide; manganese; C36H40N2 In N,N-dimethyl-formamide at 25℃; under 760.051 Torr; for 20h; regioselective reaction; | 72% |

| Conditions | Yield |
|---|---|
| With palladium on activated carbon; W(OTf)6; hydrogen In neat (no solvent) at 135℃; under 760.051 Torr; for 12h; | 98% |
| With palladium 10% on activated carbon; W(OTf)6; hydrogen at 135℃; under 760.051 Torr; for 12h; | 92% |
- 116435-51-9, 1974-05-6, 116724-27-7
3-bromoheptane

- 124-38-9
carbon dioxide

A
- 116454-37-6, 128441-06-5, 1188-02-9
2-methylheptanoic acid

B
- 124-07-2
Octanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromoheptane; carbon dioxide With nickel(II) iodide; manganese; C36H40N2 In N,N-dimethyl-formamide at 25℃; under 760.051 Torr; for 17h; Schlenk technique; Stage #2: With hydrogenchloride In water; N,N-dimethyl-formamide | A n/a B 81% |


| Conditions | Yield |
|---|---|
| With nickel(II) iodide; manganese; C36H40N2 In N,N-dimethyl-formamide at 25℃; under 760.051 Torr; for 20h; regioselective reaction; | 56% |

- 124-07-2
Octanoic acid

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; W(OTf)6; hydrogen; acetic acid at 180℃; under 22502.3 Torr; for 10h; Autoclave; | 80% |
| With palladium on activated charcoal; W(OTf)6; hydrogen; acetic acid at 180℃; under 22502.3 Torr; for 10h; Autoclave; | 80% |
- 83905-01-5Azithromycin
- 10361-37-2Barium chloride
- 593-51-1Methylamine hydrochloride
- 70775-75-61-Octanamine,N,N'-(1,10-decanediyldi-1(4H)-pyridinyl-4-ylidene)bis-, hydrochloride (1:2)
- 148553-50-8Pregabalin
- 7439-97-6Mercury
- 57-13-6Urea
- 57-11-4Stearic acid
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Consensus Reports
Specification
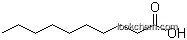
The IUPAC name of this chemical is Octanoic acid. With the CAS registry number 124-07-2 and EINECS registry number 204-677-5, it is also named as 1-Heptanecarboxylicacid. In addition, the molecular formula is C8H16O2 and the molecular weight is 144.21. It is a kind of colourless oily liquid and belongs to the classes of Miscellaneous Natural Products; Alkylcarboxylic Acids; Monofunctional & alpha,omega-Bifunctional Alkanes; Monofunctional Alkanes. And it is minimally soluble in water with a slightly unpleasant rancid-like smell and taste.
Physical properties about this chemical are: (1)ACD/LogP: 2.74; (2)ACD/LogD (pH 5.5): 1.94; (3)ACD/LogD (pH 7.4): 0.146; (4)ACD/BCF (pH 5.5): 11.324; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 117.554; (7)ACD/KOC (pH 7.4): 1.887; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 6; (11)Polar Surface Area: 37.3 Å2; (12)Index of Refraction: 1.437; (13)Molar Refractivity: 40.676 cm3; (14)Molar Volume: 155.215 cm3; (15)Polarizability: 16.125 ×10-24cm3; (16)Surface Tension: 33.075 dyne/cm; (17)Density: 0.929 g/cm3; (18)Flash Point: 107.379 °C; (19)Enthalpy of Vaporization: 50.325 kJ/mol; (20)Boiling Point: 239.33 °C at 760 mmHg; (21)Vapour Pressure: 0.022 mmHg at 25°C.
Preparation of Octanoic acid: it can be prepared by octanoyl chloride. The other product is octanoic acid octyl ester. This reaction will need reagent LiInH4 and solvent diethyl ether. The reaction should react in temperature of 0 °C for 1 hour and in the room temperature for 12 hours. The yield is about 22%.

Uses of Octanoic acid: it is used commercially in the production of esters used in perfumery and also in the manufacture of dyes. And it is also used in the treatment of some bacterial infections and used as an algaecide, bactericide, and fungicide in nurseries, greenhouses, garden centers, and interiorscapes on ornamentals. Moreover, it is also used as disinfectant in health care facilities, schools/colleges, animal care/veterinary facilities, industrial facilities, office buildings, recreational facilities. In addition, it can be used to get octan-1-ol. This reaction will need reagents Sm and 10percent HCl and solvent methanol. The reaction time is 30 minutes with ambient temperature. The yield is about 39%.

When you are using this chemical, please be cautious about it as the following:
This chemical can cause burns. During using it, wear suitable protective clothing, gloves and eye/face protection and avoid contact with eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.).
You can still convert the following datas into molecular structure:
(1)SMILES: CCCCCCCC(=O)O
(2)InChI: InChI=1/C8H16O2/c1-2-3-4-5-6-7-8(9)10/h2-7H2,1H3,(H,9,10)
(3)InChIKey: WWZKQHOCKIZLMA-UHFFFAOYAH
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 600mg/kg (600mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Acta Pharmacologica et Toxicologica. Vol. 18, Pg. 141, 1961. |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 19, Pg. 237, 1981. | |
| rat | LD50 | oral | 10080mg/kg (10080mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |