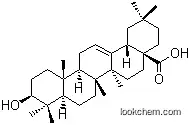
508-02-1Relevant articles and documents
A bidesmosidic oleanolic acid saponin from Panax pseudo-ginseng.
Shukla,Thakur,Pachaly
, p. 1046 - 1048 (1992)
A novel triterpenoid saponin, pseudo-ginsenoside-RI3, isolated from the rhizomes of Panax pseudo-ginseng subsp. himalaicus var. angustifolius has been characterized as 3-O-beta-D-glucopyranosyl(1----2)-beta-D-glucuronopyranosyl (1----6)-beta-D-glucopyranosyl 28-O-beta-D-xylopyranosyl-olean-12-en-28-oic acid ester by physicochemical methods.
Triterpenes from Cigarrilla mexicana
Mata, Rachel,Rios, Lizeth,del Rayo Camacho, Ma,Reguero, Ma Teresa,Lorence, David
, p. 1887 - 1889 (1988)
-From the aerial parts of Cigarrilla mexicana 3β, 23-dihydroxy-urs-12-en-28-oic acid, a new natural product, has been isolated together with the.
Oleanolic acid from antifilarial triterpene saponins of Dipterocarpus zeylanicus induces oxidative stress and apoptosis in filarial parasite Setaria digitata in?vitro
Senathilake,Karunanayake,Samarakoon,Tennekoon,de Silva,Adhikari
, p. 13 - 21 (2017)
Absence of a drug that kills adult filarial parasites remains the major challenge in eliminating human lymphatic filariasis (LF); the second leading cause of long-term and permanent disability. Thus, the discovery of novel antifilarial natural products with potent adulticidal activity is an urgent need. In the present study, methanol extracts of leaves, bark and winged seeds of Dipterocarpus zeylanicus (Dipterocarpaceae) were investigated for macro and microfilaricidal activity. Two antifilarial triterpene saponins were isolated from winged seed extracts by bioactivity guided chromatographic separation and identified using Nuclear Magnetic Resonance and mass spectroscopic analysis as oleanolic acid 3-O-β-D- glucopyranoside (1) (IC50?= 20.54 μM for adult worms, 19.71 μM for microfilariae ) and oleanolic acid 3-O-α-L-arabinopyranoside (2) (IC50?= 29.02 μM for adult worms, 25.99 μM for microfilariae). Acid hydrolysis of both compounds yielded oleanolic acid (3) which was non or least toxic to human peripheral blood mono nuclear cells (Selectivity index?= >10) while retaining similar macrofilaricidal (IC50?= 38.4 μM) and microfilaricidal (IC50?= 35.6 μM) activities. In adult female worms treated with 50 and 100 μM doses of oleanolic acid, condensation of nuclear DNA, apoptotic body formation and tissue damage was observed by using Hoechst 33342 staining, TUNEL assay and Hematoxylin and Eosin staining respectively. A dose dependent increase in caspase 3/CED3 activity and decrease in total protein content were also observed in these parasites. A dose dependant DNA fragmentation was observed in adult parasites and microfilariae. Decreased levels of reduced glutathione (GSH) and elevated levels of glutathione S transferase (GST), superoxide dismutase (SOD) and reactive oxygen species (ROS) were also observed in parasites treated with oleanolic acid indicating an oxidative stress mediated apoptotic event. Compound 3/oleanolic acid was thus identified as a potent and safe antifilarial compound in?vitro.
-
Forgacs,P.,Provost,J.
, p. 1689 (1981)
-
Antileishmanial activity of natural diterpenes from Cistus sp. and semisynthetic derivatives thereof
Fokialakis, Nikolas,Kalpoutzakis, Eleftherios,Tekwani, Babu Lal,Skaltsounis, Alexios Leandros,Duke, Stephen Oscar
, p. 1775 - 1778 (2006)
Eleven cis-clerodane diterpenes, seven labdane type diterpenes and one triterpene isolated from Cistus monspeliensis and the resin "Ladano" of Cistus creticus subsp. creticus were evaluated against Leishmania donovani promastigotes, the causative agent for visceral leishmaniasis. In addition, eleven semisynthetic manoyl oxide, seventeen labdane type derivatives and a triterpene were also evaluated for their antileishmanial activity. 18-Acetoxy-cis-clerod-3-en-15-ol, 15,18-diacetoxy-cis-clerod-3-ene and 13-(E)-8a-hydroxylabd-13-en-15-ol 2-chloroethylcarbamate exhibited the most potent and selective leishmanicidal activity with IC50 values of 3.3 μg/ml, 3.4 μg/ml and 3.5 μg/ml, respectively.
Biosynthesis of Triterpenes, Ursolic Acid and Oleanolic Acid, from Acetate in Tissue Cultures of Rabdosia japonica Hara
Seo, Shujiro,Uomori, Atsuko,Yoshimura, Yohko,Takeda, Ken'ichi,Sankawa, Ushio,et al.
, p. 1141 - 1143 (1986)
1,2-Hydride shifts in the biosynthesis of ursolic acid (2) and oleanolic acid (6), 20-H from C-19, 19-H from C-18, and 18-H from C-13 in (2) and 19-H from C-18 and 18-H from C-13 in (6), were verified in cultured cells of Rabdosia japonica Hara fed with acetate.
-
Taggart,Richter
, p. 351 (1937)
-
Araliasaponins I-XI, triterpene saponins from the roots of Aralia decaisneana
Miyase, Toshio,Shiokawa, Ken-Ichi,Zhang, Dong Ming,Ueno, Akira
, p. 1411 - 1418 (1996)
Seven new oleanane-type and four new ursane-type triterpene saponins, named araliasaponins I-XI were isolated from the roots of Aralia decaisneana, together with four known triterpene saponins. On the basis of the chemical and spectroscopic evidence, the structures of these new saponins were elucidated as follows: 3-O-β-D-xylopyranosyl-(1 → 3)-β-D-glucopyranosyl-(1 → 3)-[β-D-xylopyranosyl-(1 → 2)]-α-L-arabinopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester, 3-O-β-D-glucopyranosyl-(1 → 3)-α-L-arabinopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl ester, 3-O-β-D-glucopyranosyl-(1 → 3)-[β-D-xylopyranosyl-(1 → 2)]-α-L-arabinopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl ester, 3-O-β-D-glucopyranosyl-(1 → 3)-[β-D-xylopyranosyl-(1 → 2)-β-D-glucopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester, 3-O-β-D-glucopyranosyl-(1 → 3)-[β-D-xylopyranosyl-(1 → 2)]-β-D-galactopyranosyl oleanolic acid, 3-O-β-D-glucopyranosyl(1 → 3)-[β-D-xylopyranosyl-(1 → 2)]-β-D-galactopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester, 3-O-β-D-glucopyranosyl-(1 → 3)-[β-D-xylopyranosyl-(1 → 2)]-β-D-galactopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl(1 → 6)-β-D-glucopyranosyl ester, 3-O-β-D-glucopyranosyl-(1 → 3)-β-D-xylopyranosyl-(1 → 2)-α-L-arabinopyranosyl ursolic acid 28-O-β-D-glucopyranosyl ester, 3-O-β-D-glucopyranosyl-(1 → 3)-[β-D-xylopyranosyl-(1 → 2)]-α-L-arabinopyranosyl ursolic acid, 3-O-β-D-glucopyranosyl-(1 → 3)-α-L-arabinopyranosyl ursolic acid 28- O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl ester and 3-O-β-D-glucopyranosyl-(1 → 3)-(β-D-xylopyranosyl-(1 → 2)]-β-D-glucopyranosyl ursolic acid 28-O-β-D-glucopyranosyl ester. Copyright
Triterpenoid saponins from Albizia boromoensis Aubrv. & Pellegr
Not, Olivier Placide,Jihu, Dong,Antheaume, Cyril,Guillaume, Dominique,Pegnyemb, Dieudonn Emmanuel,Kilhoffer, Marie Claude,Lobstein, Annelise
, p. 37 - 42 (2015)
As part of our search of new bioactive saponins from Cameroonian medicinal plants, phytochemical investigation of the roots of Albizia boromoensis led to the isolation of four new oleanane-type saponins, named boromoenosides A-D (1-4). Their structures were established by direct interpretation of their spectral data, mainly HRESIMS, 1D NMR (1H, 13C NMR, and DEPT) and 2D NMR (COSY, NOESY, HSQC and HMBC), and by comparison with the literature data. All isolated saponins were assayed for their cytotoxicity against U-87 MG human glioblastoma cell lines and TG1 glioblastoma stem-like cells with no positive activity detected.
Akimaliev et al.
, (1976)
Achyranthosides A and B, novel cytotoxic saponins from Achyranthes fauriei root
Ida, Yoshiteru,Satoh, Yohko,Katoh, Mariko,Katsumata, Masumi,Nagasao, Miki,Yamaguchi, Kentaro,Kamei, Hideo,Shoji, Junzo
, p. 6887 - 6890 (1994)
Two new saponins, achyranthosides A and B, were isolated from Achyranthes fauriei root, and their structures were elucidated on the basis of chemical and physical evidences. Achyranthoside A methyl ester was found to have significant cytotoxic activity against human colon carcinoma and murine melanoma cells.
Cytotoxic triterpenoid saponins from Thalictrum atriplex
Meng, FanCheng,Wei, XiaoDong,Sun, Yan,Zeng, QingHong,Wang, Guowei,Lan, XiaoZhong,Liao, ZhiHua,Chen, Min
, p. 5757 - 5764 (2020/10/20)
Two new cycloartane glycosides, cycloatriosides A and B (1–2), and a new oleanolic acid glycoside, thaliatrioside A (3), along with 7 known triterpenoids (4–10) were isolated from Thalictrum atriplex. The structures of the new compounds were established as 3-O-β-D-galactopyranosyl (20S, 24 R)-3β,16β,25,29-tetrahydroxy-20,24-epoxycycloartane-29-O-β-D-glucopyranoside (1), 3-O-β-D-glucopyranosyl-(1→2)-α-arabinopyranosyl-3β,22ξ,30-trihydroxycycloart-24-en-21-oic acid α-L-arabinopyranosyl-(1→6)-β-D-glucopyranoside (2) and 3-O-[α-L-rhamnopyranosyl-(1→3)-β-D-xylopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranosyl]-oleanolic acid 28-O-β-D-glucopyranosyl ester (3) on the basis of extensive NMR and HR-ESI-MS analyses, along with acid hydrolysis. Their cytotoxic activities against human lung cancer cells A549 and human breast cancer cells MDA-MB-231 were evaluated using MTT method. Compound 9 showed cytotoxicity against MDA-MB-231 cell line with the IC50 value of 72.53 ± 1.08 μM.
Albidosides H and I, two new triterpene saponins from the barks of Acacia albida Del. (Mimosaceae)
Tchoukoua, Abdou,Kuiate Tabopda, Turibio,Konga Simo, Ingrid,Uesugi, Shota,Ohno, Misa,Kimura, Ken-ichi,Kwon, Eunsang,Momma, Hiroyuki,Shiono, Yoshihito,Ngadjui, Bonaventure Tchaleu
, p. 924 - 932 (2018/02/15)
Two new triterpene saponins, albidosides H (1) and I (2), along with the three known saponins were isolated from the barks of Acacia albida. Their structures were elucidated on the basis of extensive 1D- and 2D-NMR studies and mass spectrometry. Albidosid