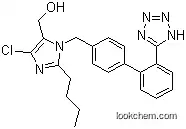
114798-26-4Relevant articles and documents
Novel and efficient debenzylation of N-benzyltetrazole derivatives with the rosenmund catalyst
Seki, Masahiko
, p. 3249 - 3255 (2014)
The Rosenmund catalyst (Pd/BaSO4) was found to efficiently catalyze debenzylation of N-benzyltetrazole derivatives with ammonium formate by catalytic transfer hydrogenation under mild conditions. The protocol has been applied to functionalized substrates to provide various angiotensin II receptor blockers in excellent yields.
Efficient synthesis of losartan, a nonpeptide angiotensin II receptor antagonist
Larsen,King,Chen,Corley,Foster,Roberts,Yang,Lieberman,Reamer,Tschaen,Verhoeven,Reider,Lo,Rossano,Brookes,Meloni,Moore,Arnett
, p. 6391 - 6394 (1994)
A highly efficient, convergent approach to the synthesis of the angiotensin II receptor antagonist losartan (1) is described. Directed ortho-metalation of 2-trityl-5-phenyltetrazole provides the key boronic acid intermediate 10 for palladium-catalyzed biaryl coupling with bromide 5 obtained from the regioselective alkylation of the chloroimidazole 2. This methodology overcomes many of the drawbacks associated with previously reported syntheses.
Unusual detritylation of tritylated tetrazole in Sartan molecules
Srimurugan, Sankareswaran,Suresh, Paulsamy,Babu, Balaji,Hiriyanna, Salmara Ganeshbhat,Pati, Hari Narayan
, p. 383 - 384 (2008)
Tritylated tetrazole of 2a underwent unusual detritylation under basic reaction condition during the synthesis of methyl ether of olmesartan medoxomil 1. The unusual detritylation was found to be a common feature in the case of all tetrazole containing Sartan molecules (3-7).
Preparation method of losartan
-
Paragraph 0033-0042, (2021/04/21)
The invention provides a preparation method of losartan, wherein the preparation method comprises the steps: reacting a nitrile intermediate shown in the specification with an azide reagent in a solvent, adding an inorganic alkali aqueous solution selected from carbonate or bicarbonate, washing, and separating out an intermediate material layer; and further separating the intermediate material layer to obtain losartan. The azide ions after the reaction can be basically and completely removed by using the conventional inorganic alkali solution, the removal effect is good, the preparation process is simple and convenient, the operation conditions are mild and easy to control, and the method is suitable for large-scale industrial production.
Method for preparing high-purity losartan
-
Paragraph 0020-0034, (2020/10/19)
The invention relates to a method for preparing high-purity losartan. The method comprises steps as follows: (1), a losartan crude product is added to an organic solvent or a mixed solvent of an organic solvent and water, and the mixture is heated to reach 20-80 DEG C and stirred; (2), the system is cooled directly or cooled after water is added or cooled to 0-5 DEG C after a part of solvent is evaporated, a material is precipitated, filtered and dried, and losartan is obtained, wherein the organic solvent used in the step (1) is any one of tetrahydrofuran, butanone, acetone and methyl alcohol or is a mixed solvent of any one of the four solvents and water. The losartan obtained with the method has high purity, any individual impurity can be reduced to 0.2% or even under 0.1%, the purity of the losartan can reach 99.5%, the cost of the method is lower, the refining yield is high, and the method is very simple in operation, environment-friendly and suitable for industrial production.
Preparation method of losartan
-
Paragraph 0054-0059, (2019/11/29)
The invention provides a preparation method of losartan. The losartan is prepared by reacting a cyano-containing intermediate as shown in a formula (I), which is described in the specification, with an azide reagent in toluene in the presence of a catalyst. After the reaction is finished, azide ions are removed through the following procedures: adding water to divide a reaction system into three layers, separating out a middle layer, adding n-butyl alcohol into the middle layer for dilution, and adding triphenylphosphine into the obtained diluted solution to remove the residual azide ions in the diluted solution. According to the preparation method, sodium nitrite is not needed, so formation of the genotoxic impurity nitrosamine is fundamentally eradicated; the obtained target losartan isgood in purity and high in yield; and the method is simple and convenient in preparation process, mild and easily controllable in operation conditions, good in safety, and suitable for large-scale industrial production.
Nickel-Catalyzed Denitrogenative ortho-Arylation of Benzotriazinones with Organic Boronic Acids: an Efficient Route to Losartan and Irbesartan Drug Molecules
Thorat, Vijaykumar H.,Upadhyay, Nitinkumar Satyadev,Cheng, Chien-Hong
, p. 4784 - 4789 (2018/11/10)
Denitrogenative ortho-arylation, vinylation and methylation of 1,2,3-benzotriazin-4-(3H)-ones with organic boronic acids catalyzed by nickel complexes to give a wide range of o-substituted benzamides were demonstrated. Further, the catalytic reaction is successfully applied to the synthesis of the popular hypertensive drugs losartan and irbesartan in high yields. (Figure presented.).
A trityl protecting group by removing method of preparing losartan medicine
-
Paragraph 0032-0039; 0047-0051, (2018/07/30)
The invention discloses a method for preparation of a Sartan drug by removal of a triphenylmethyl protective group. The method includes: under the catalysis of an insoluble weak acid, subjecting a Sartan prodrug and methanol to deprotection reaction, and after complete reaction, conducting aftertreatment to obtain the Sartan drug. The method has the characteristics of low cost, few side product, high quality product, and simple aftertreatment. At the same time, montmorillonite can be taken as insoluble weak acid, and the cost is low, thus being convenient for industrial production.
Sartan compound discoloration method
-
Paragraph 0020; 0022; 0028, (2017/07/08)
The invention relates to a sartan compound discoloration method. The method comprises the following steps: adding irbesartan or losartan crude products containing pigment impurities into solvent, and dissolving; and adding hydroboration reagent, stirring for discoloration, and crystallizing through dissociation, cooling, distillation and other means to obtain white losartan or irbesartan. The method has the advantages of mild reaction conditions, short operating cycle, high discoloration efficiency and environment friendliness, and is suitable for industrial production.
High-purity Losartan preparation method
-
Paragraph 0030-0032; 0034; 0038; 0041; 0044; 0047; 0050-0059, (2017/09/02)
The invention provides a high-purity Losartan preparation method. The method comprises the following steps: adding a catalyst having a structural formula as shown in Formula A into organic solvent, dropwisely adding acid, and stirring to perform salification; and adding a compound 1, sodium azide and tetrabutylammonium bromide into the system, heating, reacting with stirring, and performing posttreatment to obtain high-purity Losartan. The Losartan obtained by the invention is high in purity and favorable in appearance; the purity can be up to 98.4%; and the content of a single impurity can be reduced to 0.2%. The method is low in cost and high in yield; and meanwhile, the method provided by the invention is simple to operate and environment-friendly, thereby being suitable for industrial production.