59489-71-3Relevant articles and documents
A novel luciferin-based bright chemiluminescent probe for the detection of reactive oxygen species
Sekiya, Maki,Umezawa, Keitaro,Sato, Akemi,Citterio, Daniel,Suzuki, Koji
, p. 3047 - 3049 (2009)
This communication reports the synthesis, chemiluminescence properties, and biological application of KEIO-BODIPY-imidazopyrazine (KBI), a yellow-green chemiluminescent probe for the detection of reactive oxygen species (ROS) generated from living cells. The Royal Society of Chemistry 2009.
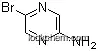
Efficient Halogenation of 2-Aminopyrazine
Grima, Josep,Lizano, Enric,Pujol, M. Dolors
, p. 2000 - 2003 (2019/10/22)
2-Aminopyrazine was halogenated with NIS, NCS, and NBS under different reaction conditions. Chlorination and bromination were achieved with good yields by using acetonitrile as the solvent. However, iodination was only obtained in poor yields. Undoubtedly, the best conditions for both mono-and dihalogenation were the use of NBS, acetonitrile, and microwave assistance for short periods. 3,5-Dibromo-2-Aminopyrazine is an excellent functionalized starting material for the synthesis of nitrogen heterocycles.
Discovery of pyrimidine nucleoside dual prodrugs and pyrazine nucleosides as novel anti-HCV agents
Guo, Shuang,Xu, Mingshuo,Guo, Qi,Zhu, Fuqiang,Jiang, Xiangrui,Xie, Yuanchao,Shen, Jingshan
, p. 748 - 759 (2019/01/26)
To explore the application potential of dual prodrug strategies in the development of anti-HCV agents, a variety of sofosbuvir derivatives with modifications at the C4 or N3 position of the uracil moiety were designed and synthesized. Some compounds exhibited potent anti-HCV activities, such as 4e and 8a–8c with similar EC50 values (0.20–0.22 μM) comparative to that of sofosbuvir (EC50 = 0.18 μM) in a genotype 1b based replicon Huh-7 cell line. Moreover, 8b displayed a good human plasma stability profile, and was easily metabolized in human liver microsomes expectantly. On the other hand, aiming to discover novel anti-HCV nucleosides, pyrazin-2(1H)-one nucleosides and their phosphoramidate prodrugs were investigated. Several active compounds were discovered, such as 25e (EC50 = 7.3 μM) and S-29b (EC50 = 19.5 μM). This kind of nucleosides were interesting and would open a new avenue for the development of antiviral agents.
NOVEL COMPOUNDS AS GPR119 AGONISTS
-
Paragraph 0589-0590, (2017/10/26)
The present invention relates to novel compounds of formula (I) as GPR119 agonist, composition compositions containing such compounds and method of preparation thereof.