53-43-0Relevant articles and documents
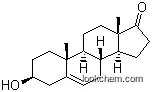
Synthesis and structure determination of 3β-hydroxyandrost-5-en-17-one (C19H28O2·CH3OH)
Verma, Rajnikant,Jasrotia, Dinesh,Sawhney, Anshu,Bhat, Mousmi,Gupta
, p. 523 - 528 (2004)
3β-Hydroxyandrost-5-en-17-one (C19H28O 2.CH3OH) has been prepared for undertaking its crystallographic analysis and to investigate the role of intra- and intermolecular interactions in steroids. The title compound crystallizes in the orthorhombic space group C2221 with unit cell parameters a = 6.7892(17), b = 12.624(2), and c = 41.136(5) A; V = 3525.7(12) A3 and Z = 8. The three-dimensional structure has been solved by direct methods. The final reliability index for the computed structure is 0.050 for 1088 observed reflections. Ring A exist in chair conformation, Ring B in half-chair conformation, and the Ring C assumes a distorted chair conformation. The five-membered Ring D adopts half-chair conformation. The A/B ring junction is quasi-trans while as B/C and C/D are trans-fused. The oxygen atom of the solvent molecule (CH3OH) is involved in O-H···O intermolecular interaction.
A steroidogenic pathway for sulfonated steroids: The metabolism of pregnenolone sulfate
Neunzig,Sánchez-Guijo,Mosa,Hartmann,Geyer,Wudy,Bernhardt
, p. 324 - 333 (2014)
In many tissues sulfonated steroids exceed the concentration of free steroids and recently they were also shown to fulfill important physiological functions. While it was previously demonstrated that cholesterol sulfate (CS) is converted by CYP11A1 to pregnenolone sulfate (PregS), further conversion of PregS has not been studied in detail. To investigate whether a steroidogenic pathway for sulfonated steroids exists similar to the one described for free steroids, we examined the interaction of PregS with CYP17A1 in a reconstituted in-vitro system. Difference spectroscopy revealed a Kd-value of 74.8 ± 4.2 μM for the CYP17A1-PregS complex, which is 2.5-fold higher compared to the CYP17A1-pregnenolone (Preg) complex. Mass spectrometry experiments proved for the first time that PregS is hydroxylated by CYP17A1 at position C17, identically to pregnenolone. A higher Km- and a lower kcat-value for CYP17A1 using PregS compared with Preg were observed, indicating a 40% reduced catalytic efficiency when using the sulfonated steroid. Furthermore, we analyzed whether the presence of cytochrome b5(b5) has an influence on the CYP17A1 dependent conversion of PregS, as was demonstrated for Preg. Interestingly, with 17OH-PregS no scission of the 17,20-carbon-carbon bond occurs, when b5is added to the reconstituted in-vitro system, while b5promotes the formation of DHEA from 17OH-Preg. When using human SOAT-HEK293 cells expressing CYP17A1 and CPR, we could confirm that PregS is metabolized to 17OH-PregS, strengthening the potential physiological meaning of a pathway for sulfonated steroids.
Etherification of Hydroxystereoids via Triflates
Belostotskii, Anatoly M.,Hassner, Alfred
, p. 5075 - 5076 (1994)
Triflates of saturated alcohols are useful in the alkylation of 3- and 17-hydroxysteroids in the presence of hindered amines.The etherification is successful even in those cases where other alkylating agents are noneffective.
Modified bile acids and androstanes—Novel promising inhibitors of human cytochrome P450 17A1
Dzichenka, Yaraslau,Shapira, Michail,Yantsevich, Aliaksei,Cherkesova, Tatsiana,Grbovi?, Ljubica,Savi?, Marina,Usanov, Sergey,Jovanovi?-?anta, Suzana
, (2020/11/17)
Cytochromes P450 are key enzymes for steroid hormone biosynthesis in human body. They are considered as targets for the screening of novel high efficient drugs. The results of screening of bile acids and androstane derivatives toward human recombinant steroid 17α-hydroxylase/17,20-lyase (CYP17A1) are presented in this paper. A group of steroids, binding with micromolar or submicromolar affinity (in a range from 9 μM – less than 0.1 μM), was identified. Results presented here showed that these steroidal compounds are able to decrease rate of hydroxylation of essential CYP17A1 substrate – progesterone, while some compounds completely inhibited enzyme activity. Structure-activity relationship (SAR) analysis based on in vitro and in silico studies showed that high affinity of the enzyme to bile acids derivatives is correlated with side chain hydrophobicity and presence of hydroxyl or keto group at C3 position. From the other side, bile acid-derived compounds with more polar side chain or substituents at C7 and C12 positions possess higher Kd values. Among androstane-derived steroids couple of Δ5-steroids with hydroxyl group at C3 position, as well as 16,17-secosteroids, were found to be high affinity ligands of this enzyme. The data obtained could be useful for the design of novel highly efficient inhibitors of CYP17A1, since the bile acids-derived compounds are for first time recognized as effective CYP17A1 inhibitors.
Deconstructive Oxygenation of Unstrained Cycloalkanamines
Han, Bing,He, Yi-Heng,Pan, Jia-Hao,Wang, Yuan-Rui,Yu, Wei,Zhang, Jian-Wu
, p. 3900 - 3904 (2020/02/11)
A deconstructive oxygenation of unstrained primary cycloalkanamines has been developed for the first time using an auto-oxidative aromatization promoted C(sp3)?C(sp3) bond cleavage strategy. This metal-free method involves the substitution reaction of cycloalkanamines with hydrazonyl chlorides and subsequent auto-oxidative annulation to in situ generate pre-aromatics, followed by N-radical-promoted ring-opening and further oxygenation by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and m-cholorperoxybenzoic acid (mCPBA). Consequently, a series of 1,2,4-triazole-containing acyclic carbonyl compounds were efficiently produced. This protocol features a one-pot operation, mild reaction conditions, high regioselectivity and ring-opening efficiency, broad substrate scope, and is compatible with alkaloids, osamines, and peptides, as well as steroids.
Method for preparing 4-androstenedione from dehydroepiandrosterone acetate
-
Paragraph 0026; 0027; 0028; 0032-0034; 0038-0040, (2019/07/04)
The invention provides a method for preparing 4-androstenedione from dehydroepiandrosterone acetate. The method comprises the following steps: carrying out a hydrolysis reaction on dehydroepiandrosterone acetate to obtain dehydroepiandrosterone, carrying out an oxidation reaction on the dehydroepiandrosterone to obtain crude 4-androstenedione, adding methanol and dichloroethane to the crude 4-androstenedione, and performing purification to obtain refined 4-androstenedione, wherein the obtained refined 4-androstenedione can be further reacted with potassium tert-butoxide to obtain 5-androstenedione. The method for preparing 4-androstenedione from dehydroepiandrosterone acetate has the following advantages: the preparation process is simple and feasible, and the production rate is improved,so the production values of enterprises are improved; and the cheap dehydroepiandrosterone acetate is used as the raw material to prepare the 4-androstenedione greatly demanded on the market, and the4-androstenedione is reacted to further prepare the 5-androstenedione, so the production cost of the enterprise is saved.
Comprehensive kinetic and substrate specificity analysis of an arylsulfatase from Helix pomatia using mass spectrometry
Correia, Mário S.P.,Ballet, Caroline,Meistermann, Hannes,Conway, Louis P.,Globisch, Daniel
, p. 955 - 962 (2019/02/09)
Sulfatases hydrolyze sulfated metabolites to their corresponding alcohols and are present in all domains of life. These enzymes have found major application in metabolic investigation of drugs, doping control analysis and recently in metabolomics. Interest in sulfatases has increased due to a link between metabolic processes involving sulfated metabolites and pathophysiological conditions in humans. Herein, we present the first comprehensive substrate specificity and kinetic analysis of the most commonly used arylsulfatase extracted from the snail Helix pomatia. In the past, this enzyme has been used in the form of a crude mixture of enzymes, however, recently we have purified this sulfatase for a new application in metabolomics-driven discovery of sulfated metabolites. To evaluate the substrate specificity of this promiscuous sulfatase, we have synthesized a series of new sulfated metabolites of diverse structure and employed a mass spectrometric assay for kinetic substrate hydrolysis evaluation. Our analysis of the purified enzyme revealed that the sulfatase has a strong preference for metabolites with a bi- or tricyclic aromatic scaffold and to a lesser extent for monocyclic aromatic phenols. This metabolite library and mass spectrometric method can be applied for the characterization of other sulfatases from humans and gut microbiota to investigate their involvement in disease development.
Synthesis of 3β-methyl ether of dehydroepiandrosterone by biotransformation of 3β-methyl ether of cholesterol with cells of mycobacteria Mycobacterium sp.
Andryushina,Stytsenko,Karpova,Yaderets,Zavarzin,Kurilov
, p. 2355 - 2358 (2020/02/18)
3p-Methyl ether of dehydroepiandrosterone was obtained by microbiological transformation of 3?-methyl ether of cholesterol with Mycobacterium sp. Androstane-3,17-dione, androst-4-ene-3,17-dione, and androsta-1,4-diene-3,17-dione were minor transformation products.
Steroid compound 3-site hydroxyl configuration inversion method
-
Paragraph 0051; 0056; 0057, (2018/12/14)
The invention discloses a steroid compound 3-site hydroxyl configuration inversion method. The method specifically comprises the following steps that (1) a steroid compound containing a 3-site hydroxyl reacts with an acyl chloride compound; (2) the product obtained in the step (1) and a substituting agent are subjected to SN2 nucleophilic substitution reaction under existing of a phase transfer catalyst; and (3) the product obtained in the step (2) is subjected to a hydrolysis reaction. Compared with a Mitsunobu method, the method does not need to use triphenylphosphine and azodiformate pricedhigher, and accordingly the production cost is greatly lowered; meanwhile, a p-nitrobenzoic acid derivative which seriously affects the water environment does not need to be used, and therefore the method is more environmentally friendly. The method adopts cesium acetate/18-crown ether-6 system to conduct 3-site hydroxyl configuration inversion, can remarkably reduce occurrence of side reactions,accordingly a higher reaction yield is obtained, and the method is finally applicable to industrialized production.
STEROIDAL COMPOUND, COMPOSITION CONTAINING THE SAME AND USE THEREOF
-
Paragraph 00861, (2018/11/21)
Provided in the present invention are a steroidal compound, a composition containing the same and a use thereof. Specifically, disclosed in the present invention are a steroidal compound as shown in formula (I) and a drug composition containing the same, or a crystal form, a pharmaceutically acceptable salt, a hydrate or solvate, a stereoisomer, a prodrug, a metabolite or an isotopic variant thereof. The compound according to the present invention can be used as a CYP17 enzyme inhibitor, and has better pharmacokinetic parameters, which can improve drug concentration of the compound in an animal, thereby improving the efficacy and safety of the drug, and in turn the compound may be applied in the preparation of the drug for treating CYP17 enzyme-related diseases (such as prostate cancer).