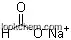Products Categories
| CAS No.: | 7757-82-6 |
|---|---|
| Name: | Sodium sulfate |
| Article Data: | 1180 |
| Molecular Structure: | |
|
|
|
| Formula: | Na2SO4 |
| Molecular Weight: | 142.043 |
| Synonyms: | anhydrous sodium sulphate;sodium carbonate powder;anhydrous sodium carbonate;sodium formate powder;sodium formaite;Sodium sulfate;sodium Sulphate;solid sodium carbonate;sodium carbonate solution;sodium formiate;anhydrous sodium formate; |
| EINECS: | 231-820-9 |
| Density: | 2.68 g/mL at 25 °C(lit.) |
| Melting Point: | 884 °C |
| Boiling Point: | 330 °C at 760 mmHg |
| Solubility: | H2O:18.5 mg/L |
| Appearance: | white crystals or powder |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 36/37/38 |
| Safety: | 24/25 |
| PSA: | 88.64000 |
| LogP: | -0.25720 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile

- 7757-82-6
sodium sulfate

| Conditions | Yield |
|---|---|
| With sulfur dioxide byproducts: S2; 600°C; | 99.98% |
| With SO2 byproducts: S2; 600°C; | 99.98% |
| With sulfur dioxide byproducts: S2; 500°C; | 66.82% |

| Conditions | Yield |
|---|---|
| In not given reaction of a soln. of (NH4)2SO4 and an excess of common salt;; | 98% |
| In not given reaction of a soln. of (NH4)2SO4 and an excess of common salt;; | 98% |
| With ammonium chloride In neat (no solvent) metathesis of (NH4)2SO4 with NaCl in presence of NH4Cl at 600 °C;; |

| Conditions | Yield |
|---|---|
| In not given in very weakly acidic soln.; | 90% |
| In not given in very weakly acidic soln.; | 90% |
- 7681-38-1
sodium hydrogen sulfate

- 7647-14-5
sodium chloride

A
- 7647-01-0
hydrogenchloride

B
- 7757-82-6
sodium sulfate

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 200 - 450°C;; | A 90% B n/a |
| In sulfuric acid |


| Conditions | Yield |
|---|---|
| In water by salting-out: addn. of a hot satd. NaCl-soln. at 82°C;; | A n/a B 90% |
| In melt High Pressure; dehydration in autoclaves: melting at 60°C, separation of pptd. Na2SO4, heating of mother-liquor to 280-300°C, further heating in an autoclave to 370°C, pptn.;; dry product;; | A n/a B >99 |
| dehydration with CBr4 or CBr4 with 10% CCl4 at about 100°C;; |

- 7757-82-6
sodium sulfate

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2O; dehydration of Glauber's salt; crystallization below 33. degree.C by additon addition of common salt or magnesium sulfate;; | 90% |
| In neat (no solvent) byproducts: H2O; dehydration of Glauber's salt; crystallization below 33. degree.C by additon addition of common salt or magnesium sulfate;; | 90% |
| In neat (no solvent) byproducts: H2O; dehydration of Glauber's salt at 100 °C;; | 46% |

| Conditions | Yield |
|---|---|
| In not given in very weakly acidic soln.; | A 20% B 80% |
| In not given in very weakly acidic soln.; | A 20% B 80% |


- 13477-34-4
calcium(II) nitrate

- 7757-83-7
sodium sulfite

B
- 7757-82-6
sodium sulfate

C
- 7632-00-0
sodium nitrite

| Conditions | Yield |
|---|---|
| In neat (no solvent) formation at heating under glowing;; | A n/a B n/a C 60% |


| Conditions | Yield |
|---|---|
| In water aq. soln. of KOH and NaOH (mol ratio 3:1) was treated with concd. H2SO4 until pH 3; pptn. with MeOH; | A 60% B 40% |


| Conditions | Yield |
|---|---|
| In not given excess of (NH4)2SO4; | A n/a B 32% |

- 12125-02-9Ammonium chloride
- 50-00-0Formaldehyde
- 9003-11-6Polyethylene-polypropylene glycol
- 121062-08-6Melanotan II
- 7647-14-5Sodium chloride
- 111-46-6Diethylene glycol
- 584-08-7Potassium carbonate
- 75-20-7Calcium carbide
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
History
The hydrate of Sodium sulfate is known as Glauber's Salt which is discovered it by the Dutch/German chemist and apothecary. He named it sal mirabilis (miraculous salt), In the 18th century, Glauber's salt began to be used as a raw material for the industrial production of soda ash (sodium carbonate), by reaction with potash (potassium carbonate). With demand for soda ash increasing , supply of Sodium sulfate (CAS NO.7757-82-6) had to increase in line. Therefore, in the 19th century, the Leblanc process, producing synthetic Sodium sulfate as a key intermediate, became the principal method of soda ash production.
Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Specification
Bisodium sulfate,with the cas number 7757-82-6, also known as Sodium sulfate, is an inorganic chemical that has several important industrial uses. Sodium sulfate is the sodium salt of sulfuric acid. When anhydrous, it is a white crystalline solid of formula Na2SO4 known as the mineral thenardite. It can be created as a byproduct during certain industrial chemical processes, but even as a "waste" product, sodium sulfate is very useful.
Physical properties about Bisodium sulfate are: (1)ACD/LogP: -1.031; (2)ACD/LogD (pH 5.5): -5.53; (3)ACD/LogD (pH 7.4): -5.53; (4)ACD/BCF (pH 5.5): 1.00; (5)ACD/BCF (pH 7.4): 1.00; (6)#H bond acceptors: 4 ; (7)#H bond donors: 2; (8)Enthalpy of Vaporization: 62.94 kJ/mol; (9)Boiling Point: 330 °C at 760 mmHg; (10)Vapour Pressure: 3.35E-05 mmHg at 25°C
Preparation of Bisodium sulfate: Bisodium sulfate is obtained from a variety of sources.
Manufacture by the Mannheim process involves the reaction of sodium chloride and sulfuric acid at very high temperatures (800 to 900 °C ).
2NaCl + H2SO4 → Na2SO4 + 2HCl
However, the majority of sodium sulfate is now obtained directly from natural salt sources. Brines with 7 to 11% sodium sulfate are used and pumped through a salt deposit to lower the solubility of the sodium sulfate so that, upon cooling, the decahydrate (Glauber's salt) will crystallize and can be separated. Heating then forms the anhydrous salt cake.
Sodium sulfate is also obtained as a by-product in the production of viscose rayon. Sulfuric acid and sodium hydroxide are used to degrade the cellulose to rayon in a fiber-spinning bath.
2NaOH + H2SO4 → Na2SO4 + 2H2O
Sodium dichromate manufacture also produces sodium sulfate as a by-product.
2Na2CrO4 + H2SO4 + H2O → Na2Cr2O7 + 2H2O + Na2SO4
Manufacture by the Hargreaves method also accounts for signifcant sodium sulfate production.
4NaCl + 2SO2 + 2H2O + O2 → 2Na2SO4 + 4HCl
Uses of Bisodium sulfate: Bisodium sulfate is mainly used for the manufacture of detergents and in the Kraft process of paper pulping. The largest use is as filler in powdered home laundry detergents, consuming approx. In the laboratory, anhydrous sodium sulfate is widely used as an inert drying agent, for removing traces of water from organic solutions. Other uses for sodium sulfate include de-frosting windows, in carpet fresheners, starch manufacture, and as an additive to cattle feed.
You can still convert the following datas into molecular structure:
(1)InChI=1S/2Na.H2O4S/c;;1-5(2,3)4/h;;(H2,1,2,3,4)/q2*+1;/p-2;
(2)InChIKey=PMZURENOXWZQFD-UHFFFAOYSA-L;
(3)SmilesS(=O)(=O)([O-])[O-].[Na+].[Na+];
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 5989mg/kg (5989mg/kg) | Shokuhin Eiseigaku Zasshi. Food Hygiene Journal. Vol. 4, Pg. 15, 1963. | |
| mouse | LDLo | intravenous | 1220mg/kg (1220mg/kg) | Compilation of LD50 Values of New Drugs. | |
| rabbit | LD50 | intravenous | 1220mg/kg (1220mg/kg) | Drugs in Japan Vol. -, Pg. 1257, 1990. |