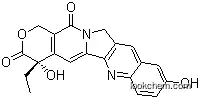
19685-09-7Relevant articles and documents
Short protecting group-free syntheses of camptothecin and 10-hydroxycamptothecin using cascade methodologies
Xu, Peng,Chen, Dong-Sheng,Xi, Jie,Yao, Zhu-Jun
, p. 976 - 981 (2015)
A convergent protecting group-free total synthesis route of camptothecin and 10-hydroxycamptothecin has been developed in this work. Cascade oxidation of 3-(hydroxymethyl)furan-2(5 H)-one and in situ intermolecular oxa Diels-Alder reaction with vinyl ether was developed and applied to construct the E-ring, and TMSCl-promoted cascade closure of the D-ring delivered the whole skeleton of the alkaloids in the total synthesis. The new short syntheses were advantageous with regard to step economy, low cost, easily available starting materials and reagents, and convenient operations.
Expeditious total syntheses of camptothecin and 10-hydroxycamptothecin
Liu, Guan-Sai,Dong, Qing-Li,Yao, Yuan-Shan,Yao, Zhu-Jun
, p. 5393 - 5396 (2008)
(Chemical Equation Presented) New expeditious total syntheses of (S)-camptothecin (16% overall yield, 95% ee) and (S)-10-hydroxycamptothecin (14% overall yield, 99% ee) have been accomplished, respectively, starting from readily available and inexpensive materials. Development, optimization, and successful application of the cascade reaction consisting of a pyrrolidine-catalyzed Michael addition, an intramolecular aldol condensation, and an oxidative aromatization, the intramolecular oxa Diels-Alder cycloaddition, and the Sharpless asymmetric dihydroxylation make these two new syntheses more efficient and straightforward.
Synthesis of 10-hydroxycamptothecin: Evaluation of new moderators for the chemoselective reduction of camptothecin
Sekhar,Anjaneyulu, Yerramilli,Acharyulu, V.R.Palle
, p. 2828 - 2834 (2011)
10-Hydroxycamptothecin is prepared by chemoselective catalytic hydrogenation of the B-ring of camptothecin over PtO2 with sulfur moderators followed by oxidation using iodobenzenediacetate. New moderators (viz. thioanisole, dimethyl sulfide, diphenyl sulfide, 2-mercapto ethanol), which moderate the hydrogenation of the B- ring of camptothecin, are being explored. Dr. Reddy's Laboratories Ltd.
Multi-gram scale synthesis of a bleomycin (BLM) carbohydrate moiety: exploring the antitumor beneficial effect of BLM disaccharide attached to 10-hydroxycamptothecine (10-HCPT)
Li, MaoLin,Huang, Weiping,Jiang, Zhilin,Shi, Yonghui,Yuan, Sisi,Fu, Kaishuo,Chen, YongJun,Zhou, Li,Zhou, Wen
, p. 6010 - 6020 (2019/04/17)
The “tumor-seeking” role of bleomycin (BLM) disaccharide has been demonstrated to serve as a promising tool for cancer diagnosis and a potential ligand for targeted therapy. However, these practical applications are often hampered by the lack of BLM disaccharide. Herein, an efficient multi-gram synthesis of peracetylated BLM disaccharide 20 is achieved by a TMSOTF-mediated glycosidation coupling manner in 43.6% overall yield in terms of benzyl galactoside. The critical innovation of the synthetic strategy is that inexpensive benzyl galactoside was first adopted to prepare an l-gulose subunit 3 as a glycosyl acceptor, with a much shorter route in 73.0% yield, and a 3-O-carbamoyl-mannose donor 4 was achieved in 47.2% yield by lowering the amount of dibutyltin oxide, and merging aminolysis and selective deacetylation into a one-pot reaction. Next, the incorporation of BLM disaccharide into 10-hydroxycamptothecin (10-HCPT), a non-specific model compound, to form conjugate 1 could significantly improve the antitumor activity and display obvious selectivity toward cancerous and normal cells in comparison with 10-HCPT. Moreover, BLM disaccharide itself was non-cytotoxic, clearly indicating the importance and potential of BLM disaccharide in solving the targeted antitumor therapy of cytotoxic drugs.
Chemical semi-synthesis process of 10-hydroxycamptothecine
-
Paragraph 0006; 0007, (2016/10/10)
The invention discloses a chemical semi-synthesis process of 10-hydroxycamptothecine. The chemical semi-synthesis process is characterized in that 20(S)-camptothecin, which is used as the raw material, is subjected to optimized N-oxidation and photochemical rearrangement reaction, and the product is subjected to silica gel column chromatography, so that high-purity 10-hydroxycamptothecine can be obtained. The chemical semi-synthesis process is simple and convenient in process conditions, the reaction reagent can be recycled, the production cost is low, the yield of 10-hydroxycamptothecine products is high, and the product is liable to separation, and accords with the national drug testing standard with the purity of 98.5%.
Total synthesis of camptothecin and SN-38
Yu, Shanbao,Huang, Qing-Qing,Luo, Yu,Lu, Wei
experimental part, p. 713 - 717 (2012/03/11)
A new practical and concise total synthesis of enantiopure camptothecin and SN-38 (14% overall yield, 99.9% ee and 99.9% purity) was described, starting from inexpensive and readily available materials. The development of column chromatography-free purification was achieved in all steps, which offers an economic industrial process to the camptothecin-family alkaloids.
Benzyl ether-linked glucuronide derivative of 10-hydroxycamptothecin designed for selective camptothecin-based anticancer therapy
Leu, Yu-Ling,Chen, Chien-Shu,Wu, Yih-Jang,Chern, Ji-Wang
, p. 1740 - 1746 (2008/09/21)
A β-glucuronidase-activated prodrug approach was applied to 10-hydroxycamptothecin, a Camptotheca alkaloid with promising antitumor activity but poor water solubility. We synthesized a glucuronide prodrug of 10-hydroxycamptothecin (7) in which glucuronic acid is connected via a self-immolative 3-nitrobenzyl ether linker to the 10-OH group of 10-hydroxycamptothecin. Compound 7 was 80 times more soluble than 10-hydroxycamptothecin in aqueous solution at pH 4.0 and was stable in human plasma. Prodrug 7 was 10-to 15-fold less toxic than the parent drug to four human tumor cell lines. In the presence of β-glucuronidase, prodrug 7 could be activated to elicit similar cytotoxicity to the parent drug in tumor cells. Enzyme kinetic studies showed that Escherichia coli β-glucuronidase had a quite low Km of 0.18 μM for compound 7 and exhibited 520 times higher catalytic efficiency for 7 than for 6 (a glucuronide prodrug of 9-aminocamptothecin). Molecular modeling studies predicted that compound 7 would have a higher binding affinity to human β-glucuronidase than compound 6. Prodrug 7 may be useful for selective cancer chemotherapy by a prodrug monotherapy (PMT) or antibody-directed enzyme prodrug therapy (ADEPT) strategy.
PROCESS FOR PREPARING IRINOTECAN
-
Page/Page column 6-7, (2008/06/13)
The present invention relates to a process for the preparation of pure irinotecan or salts thereof, and a process for the preparation of intermediate compound 7-ethyl-10-hydroxycamptothecin.
PROCESS FOR PREPARING TOPOTECAN
-
Page/Page column title page; 7, (2008/06/13)
A process for preparing topotecan.
A practical regiospecific synthesis of 9-nitrocamptothecin
Fu, Qingquan,Chen, Zhiyong
, p. 1940 - 1942 (2007/10/03)
9-Nitrocamptothecin has shown potent antitumor activity against many types of human cancers. A practical scale-up procedure for this compound is reported by selective reduction of corresponding sulfonate. Georg Thieme Verlag Stuttgart.