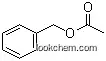
140-11-4Relevant articles and documents
Preparation of ammonium cerium phosphate via low-heating solid state reaction and its catalysis for benzyl acetate synthesis
Liao, Sen,Chen, Zhipeng,Liu, Gang,Tian, Xiaozhen,Wang, Tianshun,Wu, Wenwei
, p. 378 - 382 (2010)
Ammonium cerium phosphate was prepared with (NH4)3PO4?3H2O and Ce(SO4)2?4H2O as raw materials and PEG-400 as surfactant via a solid state reaction at low-heating temperature. The characterization result of XRD indicates that the molecular formula of the product was (NH4)2Ce(PO4)2?H2O. The synthesis of benzyl acetate was carried out with H2SO4/ammonium cerium phosphate as catalyst, and uniform experimental design as well as data mining technology was applied to the experiments, in which the effect of the reaction time, the molar ratio of acid to alcohol and the amount of catalyst on the conversion yield of acetic acid were studied. When benzalcohol was 0.10 mol, under the optimal reaction conditions, i.e. reaction time of 174 min, 2.02 of molar ratio of acid to alcohol and 0.5 g of catalyst, the esterification rate of acetic acid was 97.9%. The ammonium cerium phosphate had potential for industry application since it not only was feasible and simple in synthesis technics, but also had good catalysis activity for the synthesis of benzyl acetate.
Organocatalysis by site-isolated N-heterocyclic carbenes doped into the UIO-67 framework
Schumacher, William T.,Mathews, Madeleine J.,Larson, Sean A.,Lemmon, Carl E.,Campbell, Karin A.,Crabb, Brendan T.,Chicoine, Brent J.-A.,Beauvais, Laurance G.,Perry, Marc C.
, p. 422 - 427 (2016)
Two imidazolium-tagged biphenyldicarboxylates were synthesized and incorporated into the UiO-67 framework. Post synthetic exchange did not prove a useful route to these materials. Alternatively, mixed-linker synthesis of the solid afforded materials loaded with 6-7% imidazolium linker, corresponding to fewer than one active site per cage. Powder X-ray diffraction patterns for the doped metal-organic frameworks (MOFs) revealed that they were isostructural with UiO-67. Once activated, the N-heterocylic carbene (NHC) containing MOF was found to catalyze the transesterification of vinyl acetate with benzyl alcohol in good yield. The catalyst could be recycled with a modest drop in conversion. This is the first report of a NHC-doped MOF acting as an organocatalyst.
Asymmetric syntheses of 8-oxabicyclo[3,2,1]octane and 11-oxatricyclo[5.3.1.0]undecane from glycals
Liao, Hongze,Leng, Wei-Lin,Le Mai Hoang, Kim,Yao, Hui,He, Jingxi,Voo, Amanda Ying Hui,Liu, Xue-Wei
, p. 6656 - 6661 (2017)
Herein, we describe an efficient method to prepare enantiomerically pure 8-oxabicyclo[3.2.1]octanes via gold(i)-catalyzed tandem 1,3-acyloxy migration/Ferrier rearrangement of glycal derived 1,6-enyne bearing propargylic carboxylates. The resultant compou
Finding the Switch: Turning a baeyer-villiger monooxygenase into a NADPH Oxidase
Brondani, Patrcia B.,Dudek, Hanna M.,Martinoli, Christian,Mattevi, Andrea,Fraaije, Marco W.
, p. 16966 - 16969 (2014)
By a targeted enzyme engineering approach, we were able to create an efficient NADPH oxidase from a monooxygenase. Intriguingly, replacement of only one specific single amino acid was sufficient for such a monooxygenase-to-oxidase switch - a complete transition in enzyme activity. Pre-steady-state kinetic analysis and elucidation of the crystal structure of the C65D PAMO mutant revealed that the mutation introduces small changes near the flavin cofactor, resulting in a rapid decay of the peroxyflavin intermediate. The engineered biocatalyst was shown to be a thermostable, solvent tolerant, and effective cofactor-regenerating biocatalyst. Therefore, it represents a valuable new biocatalytic tool.
Inline Reaction Monitoring of Amine-Catalyzed Acetylation of Benzyl Alcohol Using a Microfluidic Stripline Nuclear Magnetic Resonance Setup
Oosthoek-De Vries, Anna Jo,Nieuwland, Pieter J.,Bart, Jacob,Koch, Kaspar,Janssen, Johannes W. G.,Van Bentum, P. Jan M.,Rutjes, Floris P. J. T.,Gardeniers, Han J. G. E.,Kentgens, Arno P. M.
, p. 5369 - 5380 (2019)
We present an in-depth study of the acetylation of benzyl alcohol in the presence of N,N-diisopropylethylamine (DIPEA) by nuclear magnetic resonance (NMR) monitoring of the reaction from 1.5 s to several minutes. We have adapted the NMR setup to be compatible to microreactor technology, scaling down the typical sample volume of commercial NMR probes (500 μL) to a microfluidic stripline setup with 150 nL detection volume. Inline spectra are obtained to monitor the kinetics and unravel the reaction mechanism of this industrially relevant reaction. The experiments are combined with conventional 2D NMR measurements to identify the reaction products. In addition, we replace DIPEA with triethylamine and pyridine to validate the reaction mechanism for different amine catalysts. In all three acetylation reactions, we find that the acetyl ammonium ion is a key intermediate. The formation of ketene is observed during the first minutes of the reaction when tertiary amines were present. The pyridine-catalyzed reaction proceeds via a different mechanism.
Electrochemical reduction of methyl 2-bromomethylbenzoate at carbon cathodes in dimethylformamide containingwater
Allen, Caroline R.,Brown, Drew K.,Potts, Jessica L.,Ji, Chang
, p. G3069-G3072 (2013)
Cyclic voltammetry and controlled-potential electrolysis have been employed to examine the electrochemical reduction of methyl 2-bromomethylbenzoate at carbon cathodes in dimethylformamide (DMF) containing tetramethylammonium tetrafluoroborate (TMABF4). A cyclic voltammogram for the reduction of the substrate exhibits one irreversible cathodic wave with a peak potential of -1.45 V vs. SCE, which is due to the two-electron cleavage of the benzylic carbon-bromine bond. The corresponding reductive peak current also increases incrementally with the amount of water in DMF. Bulk electrolyses of methyl 2-bromomethylbenzoate have been carried out at -1.85 V vs. SCE with different concentrations of water in the solvent. The reduction process involves carbanion intermediates to afford various products including phthalide, which is generated via intramolecular cyclization that is affected by the presence of water. Detailed mechanism for the electrochemical reaction is proposed and further studied by isotope incorporation experiment.
P-Cymenesulphonic acid: An organic acid synthesised from citrus waste
Clark, James H.,Fitzpatrick, Emma M.,MacQuarrie, Duncan J.,Pfaltzgraff, Lucie A.,Sherwood, James
, p. 144 - 149 (2012)
An organic acid, p-cymene-2-sulphonic acid, is synthesised from citrus waste and demonstrated to be comparable to p-toluenesulphonic acid in examples of acid catalysis. Firstly the essential oil found in citrus waste is extracted by either steam distillation or microwave irradiation. Oxidising the limonene in the citrus oil to p-cymene followed by sulphonation gives p-cymene-2-sulphonic acid.
Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads
Melo, Ana D. Q.,Silva, Francisco F. M.,Dos Santos, José C. S.,Fernández-Lafuente, Roberto,Lemos, Telma L. G.,Dias Filho, Francisco A.
, (2017)
Enzymes serve as biocatalysts for innumerable important reactions, however, their application has limitations, which can in many cases be overcome by using appropriate immobilization strategies. Here, a new support for immobilizing enzymes is proposed. This hybrid organic-inorganic support is composed of chitosan—a natural, nontoxic, biodegradable, and edible biopolymer—and sodium polyphosphate as the inorganic component. Lipase B from Candida antarctica (CALB) was immobilized on microspheres by encapsulation using these polymers. The characterization of the composites (by infrared spectroscopy, thermogravimetric analysis, and confocal Raman microscopy) confirmed the hybrid nature of the support, whose external part consisted of polyphosphate and core was composed of chitosan. The immobilized enzyme had the following advantages: possibility of enzyme reuse, easy biocatalyst recovery, increased resistance to variations in temperature (activity declined from 60?C and the enzyme was inactivated at 80 ?C), and increased catalytic activity in the transesterification reactions. The encapsulated enzymes were utilized as biocatalysts for transesterification reactions to produce the compound responsible for the aroma of jasmine.
Ultrasound assisted heteropoly acid catalyst SiW12/SiO 2 for synthesis of benzyl acetate
Chen, Xi,Wang, Jun,Han, Yue,Lu, Xiao-Ping,Han, Ping-Fang
, p. 623 - 627 (2013)
Under ultrasonic radiation, benzyl acetate is synthesized from benzyl alcohol and glacial acetic acid in presence of catalyst SiW12/ SiO2. The catalyst was prepared and characterized by IR, X-ray diffraction and SEM in the experiment. The XRD result indicates that heteropoly acid retains the Keggin-type structure. The kinetic parameters of reaction were, respectively measured at 90, 100 and 110 °C and the kinetic equation was built. Then, the effects of reaction temperature, the amount of catalyst, molar ratio of benzyl alcohol to glacial acetic acid and ultrasonic intensity on the esterification yield were discussed. The results showed that the optimum operating parameters for the present work when 10 kHz ultrasonic frequency and 1.0 W/cm2 ultrasonic intensity are as follows: reaction temperature: 110 °C, use amount of catalyst: 1.5 g, molar ratio of benzyl alcohol to glacial acetic acid: 1.5; reaction time: 75 min. Under such conditions, the esterification yield of the reaction reached above 95.3 %.
The Darbeau-White-Gibble reaction: An N-nitrosoamide-mediated Ritter-type reaction. Part I. A study of electronic, steric, and orbital effects in the nucleophile1a
Darbeau,Gibble,Pease,Bridges,Siso,Heurtin
, p. 1084 - 1090 (2001)
Benzyl cations were generated via thermal decomposition of N-benzyl-N-nitrosopivalamide in molten 4-R-substituted benzonitriles (R = NH2, Me2N, MeO, Me, H, F, and CF3). In each case, the benzyl cation was intercepted competitively by pivalate ion to yield benzyl pivalate and by the benzonitriles to yield the corresponding N-4-R-benzonitrilium ion. The latter onium ions reacted with pivalate ion to form benzimidic anhydrides which rearranged to yield N-4-R-benzoyl-N-pivaloylbenzylamines (i.e. unsymmetrical diacylamines). The yield of diacylamines (maximum ~10.6% for R = H) is smaller than from the corresponding reactions in acetonitrile and varied systematically with the nature and location of the R group on the aromatic nucleus. Both electron-releasing and electron-withdrawing groups at the para position effected a diminution of the yield of diacylamine; indeed for R = NH2, no diacylamine was formed. ortho Substitution of the aromatic nucleus resulted in significantly diminished yields of diacylamine, as did nucleophilic attack on the nitrilium ion by pivalate rather than acetate. Thus, both electronic and steric effects in nucleophilic attack on the nitrilium carbon were observed. The ratios of counterion-derived product to solvent-derived product for both the first-formed benzyl cation and the less reactive benzonitrilium ion are similar. This observation is interpreted in terms of the intermediacy of nitrogenous entity-separated ion-pairs in these deaminations.
 Xi:Irritant;
Xi:Irritant;



