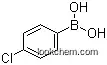
1679-18-1Relevant articles and documents
SYNTHESIS OF ARYLBORONIC ACIDS VIA THE REACTION OF BORANE WITH ARYLMAGNESIUM HALIDES
Kabalka, George W.,Sastry, Usha,Sastry, K.A.R.,Knapp, Furn F.,Srivastava, Prem C.
, p. 269 - 274 (1983)
The reaction of borane complexes with arylmagnesium halides produces the corresponding arylborohydrides in high yield.The arylborohydrides are readily hydrolyzed to the arylboronic acids.The syntheses are conveniently carried out in one pot.The reaction mechanism was clarified via a boron-11 NMR study.
Aryl boronic acid preparation method
-
Paragraph 0033-0036; 0041, (2020/01/25)
The invention belongs to the technical field of fine chemical engineering, and relates to an aryl boronic acid preparation method. In the prior art, aryl boronic acid as a novel safe and environmentally-friendly arylation reagent is widely used in scientific research and production of various fine chemicals containing aryl structures in the fields of medicines, pesticides, advanced materials and the like; and the aryl boronic acid compound preparation method reported in the disclosed literature has problems of harsh reaction conditions and high cost. A purpose of the invention is to provide amethod, wherein an aryl boron compound is formed by carrying out a reaction on a Grignard reagent and trialkyl borate under mild conditions, the composition of the aryl boron compound is converted from the main component diaryl borate into the main component aryl borate, and the aryl borate is hydrolyzed to obtain aryl boric acid, so that the preparation cost of the acyl aryl boric acid compound can be remarkably reduced, and the method has good practical application prospect.
Synthesis method of P-chlorophenylboronic acid
-
Paragraph 0022-0029, (2020/04/22)
The invention belongs to the technical field of organic synthesis, and particularly relates to a synthesis method of p-chlorophenylboronic acid, which comprises the following steps: carrying out Friedel-Crafts reaction between chlorobenzene used as a raw material and boron trichloride under the action of a catalyst to obtain dichloro(4-chlorophenyl) borane, and carrying out acidic hydrolysis to obtain p-chlorophenylboronic acid. According to the method, p-chlorophenylboronic acid is synthesized by taking chlorobenzene as a raw material through a Friedel-Crafts reaction, and the method has theadvantages of novel synthesis route, simple and practical operation, easily available raw materials, low cost, environmental friendliness, and the like.