Bulk Drug Intermediates are integral to the pharmaceutical manufacturing process. These chemical compounds are produced on a massive scale, serving as foundational building blocks in the creation of pharmaceuticals. They play a crucial role in the synthesis of Active Pharmaceutical Ingredients (APIs) or the final drug product, enabling cost-effective and efficient production.
Quality control is paramount in the production of bulk intermediates. Maintaining high purity levels and eliminating contaminants is essential to ensure the safety and efficacy of the final pharmaceutical products. Stringent quality control measures, including adherence to Good Manufacturing Practices (GMP), are standard practice.
The regulatory landscape for bulk drug intermediates is rigorous. Regulatory authorities like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) enforce strict guidelines. Compliance with these regulations is not only necessary but also essential to uphold product quality and safety.
Bulk intermediates can be commercially available or custom-synthesized by pharmaceutical companies or contract manufacturing organizations (CMOs). Custom synthesis allows for the tailoring of intermediates to the specific requirements of a drug's formulation.
Safety is a paramount concern in working with bulk intermediates. Due to the potential involvement of hazardous chemicals and processes, strict safety protocols must be followed to protect both workers and the environment.
The synthesis of bulk intermediates can also be a significant component of a pharmaceutical company's intellectual property portfolio, often protected by patents.
In conclusion, bulk drug intermediates are vital components of pharmaceutical manufacturing, produced on a large scale to form the foundation of pharmaceutical production. Their quality, purity, and adherence to regulatory standards are central to ensuring the safety and effectiveness of pharmaceutical products.
![methyl 3-({[(1,1-dimethylethyl)oxy]carbonyl}amino)-5-iodo-2-thiophenecarboxylate](http://file1.lookchem.com/300w/substances/2022-03-17-06/c8f1b0c0-0833-4168-9086-aace0c39a3aa.png)
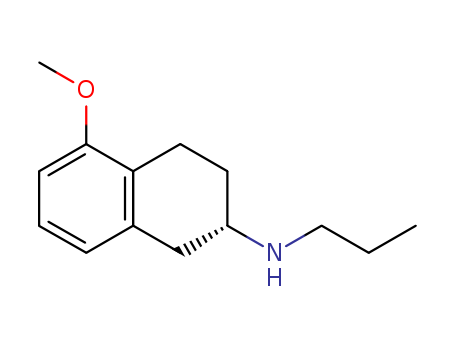
![6-Bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-d]pyrimidin-7(8H)-one](http://file1.lookchem.com/300w/synthetic/2022-01-23-05/9607f6f6-1355-4175-94fb-586911b297d9.png)
![2H-1-Benzopyran-2-one,3,3'-[[4-(dimethylamino)phenyl]methylene]bis[4-hydroxy-](http://file1.lookchem.com/cas/reactions/2021/07/12/1274931.png)
![N-Benzoyl-5'-O-(4,4-Dimethoxytrityl)-2'-O-[(tert-butyl)dimethylsilyl]adenosine-3'-(2-cyanoethyl-N,N-diisopropyl)phosphoramidite](http://file1.lookchem.com/300w/synthetic/2022-01-31-06/2df3ea65-a6b4-497f-bbd5-986456507126.png)
![5-(4-(1-(imidazo[1,2-a]pyridin-7-yl)ethyl)piperazin-1-yl)-1,3,4-thiadiazol-2-amine](http://file1.lookchem.com/300w/substances/2022-04-20-10/e611ec83-2e41-4acc-bdbd-301ced4b8f20.png)
![7-Amino-8-oxo-3-(cis-prop-1-enyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid diphenylmethyl ester hydrochloride](http://file1.lookchem.com/300w/substances/2022-04-06-09/a36aada6-b806-4834-ae5a-701f3d357ff1.png)
![(1S,2R,3S,5R)-3-(Phenymethyloxy)-2-(phenylmethoxy)methyl-6-oxabicyclo[3.1.0]hexane](http://file1.lookchem.com/cas/reactions/2021/07/01/3554957.png)
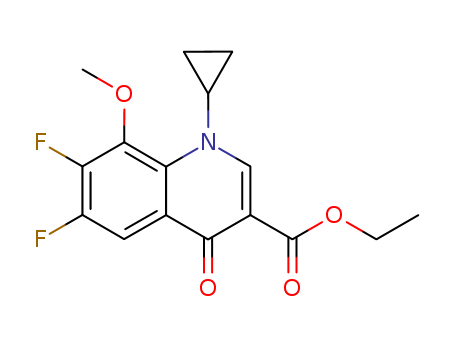
![N-{3-[5-(2-chloro-4-pyriMidinyl)-2-(1,1-diethylethyl)-1,3-thiazol-4-yl]-2-fluoraphenyl}-2,6-difluorobenzenesulfonaMide](http://file1.lookchem.com/cas/reactions/2021/07/06/19689982.png)
![3-Azabicyclo[3.1.0]hexane-1,3-dicarboxylic acid, 3-(1,1-dimethylethyl) 1-ethyl](http://file1.lookchem.com/300w/substances/2022-04-07-07/14a75844-8226-4c46-adf3-41dcb00f8ea3.png)
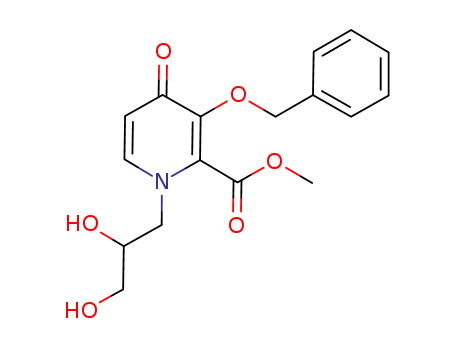
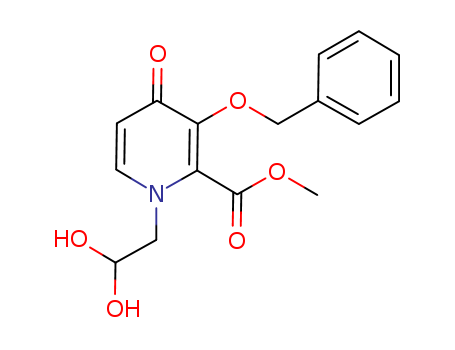
![(4R,12aS)-7-(benzyloxy)-4-Methyl-3,4-dihydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-6,8(12H,12aH)-dione](http://file1.lookchem.com/cas/reactions/2021/06/29/20386335.png)
![(4R,12aS)-7-(benzyloxy)-9-broMo-4-Methyl-3,4-dihydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-6,8(12H,12aH)-dione](http://file1.lookchem.com/300w/synthetic/2022-01-31-06/30bb8aba-7a29-456d-9a4f-442dd1599a37.png)
![(4R,12aS)-N-(2,4-difluorobenzyl)-7-benzylhydroxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxaMide](http://file1.lookchem.com/cas/reactions/2021/06/29/20386343.png)
![8H-7,10-Methanocyclopropa[18,19][1,10,3,6]dioxadiazacyclononadecino[11,12-b]quinoxaline-8-carboxylic acid, 5-(1,1-diMethylethyl)-1,1a,3,4,5,6,9,10,18,19,20,21,22,22a-tetradecahydro-14-Methoxy-3,6-dioxo-, Methyl ester, (1aR,5S,8S,10R,22aR)-](http://file1.lookchem.com/300w/synthetic/2022-01-23-03/e08dee3f-687d-41e3-983e-b1fa77e0f806.png)